Intentional heterogeneity in autologous cell-based gene therapies: strategic considerations for first-in-human trials
- PMID: 40480658
- PMCID: PMC12142127
- DOI: 10.1136/jitc-2024-011301
Intentional heterogeneity in autologous cell-based gene therapies: strategic considerations for first-in-human trials
Abstract
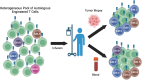
Cell-based gene therapies, including chimeric antigen receptor-T, T-cell receptor-T, and tumor-infiltrating lymphocyte therapies, have transformed the treatment landscape for certain cancers, yet their efficacy in solid tumors remains limited. Next-generation therapies aim to overcome biological barriers, enhance potency and safety, and streamline development timelines through innovative approaches. Recent advances in genome editing technologies have identified hundreds of gene edits that improve T-cell functionality in preclinical models. However, the limited direct translatability of these findings and the impracticality of testing each of the individual edits in a traditional clinical trial highlight the need for more efficient strategies.This article provides an overview of genome-wide screens that identify gene knockouts and knock-ins to enhance T-cell function and the limitations with translating these results to human trials. Next, we propose a novel clinical trial design for testing multiple gene modifications simultaneously within a single T-cell infusion product. This approach would enable head-to-head evaluation of edits in an internally controlled setting, accelerating the identification of promising candidate edits. Key considerations for Chemistry, Manufacturing, and Controls, non-clinical evaluation, and clinical protocols are discussed, with an emphasis on patient safety and ethical transparency.This framework is informed by insights shared at the "Unlocking Complex Cell-based Gene Therapies" workshop, held on May 6, 2024. Co-hosted by Friends of Cancer Research and the Parker Institute for Cancer Immunotherapy, the event brought together participants from academia, the US Food and Drug Administration, and patient advocacy groups. By fostering collaboration among these stakeholders, this innovative approach aims to accelerate the development of effective cell-based therapies for complex diseases.
Keywords: Adoptive cell therapy - ACT; Chimeric antigen receptor - CAR; Gene therapy; Solid tumor; T cell Receptor - TCR.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JEC is a cofounder of Twain Therapeutics, director at Dispatch Therapeutics and 3T Biosciences, and receives research funding from Janssen. PDG is a founder and scientific advisory board (SAB) member of, has equity in, and is receiving research support from Affini-T Therapeutics; is an SAB member of and has equity in Immunoscape, RAPT Therapeutics, Earli, Metagenomi, Nextech, and Catalio. CLM is an inventor on multiple patents related to CAR-T cell therapy and has received royalties for licenses to Juno Therapeutics and CARGO Therapeutics; is a co-founder of Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-T cells and holds equity in these companies; is a consultant for CARGO, Link, Immatics, Ensoma, Adaptimmune and received research funding from Lyell and Tune Therapeutics. CHJ is an inventor on patents and/or patent applications licensed to Novartis and receives license revenue from such licenses; is a scientific founder of Tmunity Therapeutics, Dispatch Biotherapeutics, Bluewhale Bio, Marble Therapeutics and Capstan Therapeutics; is a member of the scientific advisory boards of AC Immune, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Kite Gilead, Poseida, Replay Bio, Treadwell Therapeutics, Verismo, Viracta, Vittoria and WIRB-Copernicus; and receives sponsored research support from Kite Gilead. AM is a co-founder of Site Tx, Arsenal Biosciences, Spotlight Therapeutics, and Survey Genomics; serves on the boards of directors of Site Tx, Spotlight Therapeutics, and Survey Genomics; is a member of the scientific advisory boards of Site Tx, Arsenal Biosciences, Cellanome, Spotlight Therapeutics, Survey Genomics, NewLimit, Amgen, and Tenaya; owns stock in Arsenal Biosciences, Site Tx, Cellanome, Spotlight Therapeutics, NewLimit, Survey Genomics, Tenaya, and Lightcast; has received fees from Site Tx, Arsenal Biosciences, Cellanome, Spotlight Therapeutics, NewLimit, Gilead, Pfizer, 23andMe, PACT Pharma, Juno Therapeutics, Tenaya, Lightcast, Trizell, Vertex, Merck, Amgen, Genentech, GLG, ClearView Healthcare, AlphaSights, Rupert Case Management, Bernstein, and ALDA; is an investor in and informal advisor to Offline Ventures and a client of EPIQ; has received research support from the Parker Institute for Cancer Immunotherapy, the Emerson Collective, Arc Institute, Juno Therapeutics, Epinomics, Sanofi, GlaxoSmithKline, Gilead, and Anthem and reagents from Genscript and Illumina. MVM is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and the University of Pennsylvania (some licensed to Novartis); reports grant or research support from Kite Pharma and Moderna; reports consulting fees from several companies involved in cell therapies; has equity in 2Seventy Bio, A2Bio, Cargo, Century Therapeutics, Neximmune, Oncternal and TCR2; and has served on the board of directors for 2Seventy Bio. AR reports personal/consulting fees from Amgen, Arcus, Bioncotech, Bristol Myers Squibb Company, Chugai, Compugen, Cytomex, Five Prime, FLX-Bio, Genentech/Roche, MapKure, Merck, Merus, Rgenix, PACT Pharma, Synthekine, and Tango Therapeutics; support for other professional activities from Arsenal Bio; is a fiduciary officer at Apricity Health, Arcus Bio, and Lutris Pharma; holds a patent with Arsenal Bio; and owns stock or stock options in Appia, Apricity Health, Arcus Bio, Bioncotech, Compugen, Cytomex, Five Prime, FLX-Bio, Immpact Bio, Highlight Therapeutics, Inspirina, Lutris Pharma MapKure, Merus, Rgenix, PACT Pharma, Synthekine, and Tango Therapeutics all outside the submitted work. CRC, EY, MDS, JDA, and UD report no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
