The electroencephalography protocol for the Accelerating Medicines Partnership® Schizophrenia Program: Reliability and stability of measures
- PMID: 40480970
- PMCID: PMC12144291
- DOI: 10.1038/s41537-025-00622-0
The electroencephalography protocol for the Accelerating Medicines Partnership® Schizophrenia Program: Reliability and stability of measures
Abstract
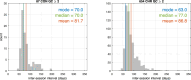
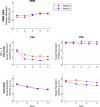
Individuals at clinical high risk for psychosis (CHR) have variable clinical outcomes and low conversion rates, limiting development of novel and personalized treatments. Moreover, given risks of antipsychotic drugs, safer effective medications for CHR individuals are needed. The Accelerating Medicines Partnership® Schizophrenia (AMP® SCZ) Program was launched to address this need. Based on past CHR and schizophrenia studies, AMP SCZ assessed electroencephalography (EEG)-based event-related potential (ERP), event-related oscillation (ERO), and resting EEG power spectral density (PSD) measures, including mismatch negativity (MMN), auditory and visual P300 to target (P3b) and novel (P3a) stimuli, 40-Hz auditory steady state response, and resting EEG PSD for traditional frequency bands (eyes open/closed). Here, in an interim analysis of AMP SCZ EEG measures, we assess test-retest reliability and stability over sessions (baseline, month-2 follow-up) in CHR (n = 654) and community control (CON; n = 87) participants. Reliability was calculated as Generalizability (G)-coefficients, and changes over session were assessed with paired t-tests. G-coefficients were generally good to excellent in both groups (CHR: mean = 0.72, range = 0.49-0.85; CON: mean = 0.71, range = 0.44-0.89). Measure magnitudes significantly (p < 0.001) decreased over session (MMN, auditory and visual target P3b, visual novel P3a, 40-Hz ASSR) and/or over runs within sessions (MMN, auditory/visual novel P3a and target P3b), consistent with habituation effects. Despite these small systematic habituation effects, test-retest reliabilities of the AMP SCZ EEG-based measures are sufficiently strong to support their use in CHR studies as potential predictors of clinical outcomes, markers of illness progression, and/or target engagement or secondary outcome measures in controlled clinical trials.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare the following competing interests: B.H.E. is part of the Advisory Board of Boehringer Ingelheim, Lundbeck Pharma A/S; and has received lecture fees from Boehringer Ingelheim, Otsuka Pharma Scandinavia AB, and Lundbeck Pharma A/S; C.A. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda; C.D.C. has received grant support from Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation and honoraria or travel support from Angelini, Janssen, and Viatris; C.M.C. is an Associate Editor of Nature: Schizophrenia; D.D. has received honorary funds for one educational seminar for CSL Sequiris; D.O.P. has been a consultant to Alkermes; E.Y.H.C. has received speaker fees at non-promotional educational events; GAL has been a consultant for Bristol Myers Squibb, Cerevel, Johnson & Johnson, Neurocrine, NeuroSig, and Sosei-Heptares; J.K. has received speaking or consulting fees from Janssen, Boehringer Ingelheim, ROVI and Lundbeck; J.M.K. is a consultant to or receives honoraria and/or travel support and/or speakers fees from Alkermes, Allergan, Boehringer-Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HealthRhythms, HLS Therapeutics, Indivior, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, Karuna Therapeutics/Bristol Myers Squibb, LB Pharmaceuticals, Mapi, Maplight, Merck, Minerva, Neurocrine, Newron, Novartis, NW PharmaTech, Otsuka, Roche, Saladax, Sunovion, and Teva; and is on Advisory Boards for Alkermes, Boehringer-Ingelheim, Cerevel, Click Therapeutics, Karuna/BMS, Lundbeck, Merck, Newron, Novartis, Otsuka, Sumitomo, Teva, and Terran; and has received grant support from Lundbeck, Janssen, Otsuka, and Sunovion; and has shareholder interest in Cerevel (public/stock), HealthRhythms (private/stock options), Karuna/BMS (public), LB Pharmaceuticals (private/stock options), North Shore Therapeutics (private/stock), and Vanguard Research Group (private/40% owner); P.F.P. has received research funds or personal fees from Lundbeck, Angelini, Menarini, Sunovion, Boehringer Ingelheim, Proxymm Science, Otsuka, outside the current study; R.S.K. has been a consultant to Alkermes and Boehringer-Ingelheim; R.U. has received speaker fees at non-promotional educational event: Otsuka: Consultancy for Viatris and Springer Healthcare. Honorary General Secretary British Association for Psychopharmacology (unpaid); S.W.W. has received speaking fees from the American Psychiatric Association and from Medscape Features. He has been granted US patent No. 8492418 B2 for a method of treating prodromal schizophrenia with glycine agonists. He owns stock in NW PharmaTech; and Z.T. has been a consultant for Manifest Technologies. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

