GAS6/AXL signaling promotes M2 microglia efferocytosis to alleviate neuroinflammation in sepsis-associated encephalopathy
- PMID: 40480981
- PMCID: PMC12144116
- DOI: 10.1038/s41420-025-02507-8
GAS6/AXL signaling promotes M2 microglia efferocytosis to alleviate neuroinflammation in sepsis-associated encephalopathy
Abstract
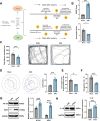
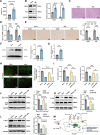
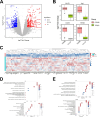
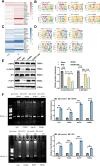
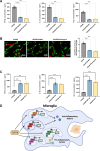
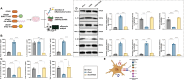
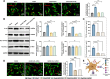
Sepsis-associated encephalopathy (SAE) is a severe complication marked by acute central nervous system (CNS) injury and neuroinflammation. M2 microglia efferocytosis is essential for resolving neuroinflammation, but its regulatory mechanisms remain unclear. This study explored the GAS6/AXL signaling pathway in SAE, hypothesizing its role in enhancing anti-inflammatory responses and efferocytosis. A mouse model of SAE was established via cecal ligation and puncture (CLP), and cognitive impairments were assessed through behavioral tests. Brain tissues and microglia were isolated for RNA sequencing (RNA-Seq) to identify genes associated with the GAS6/AXL pathway. Recombinant GAS6 (rGAS6) protein and an AXL inhibitor were used to examine the pathway's effects on microglial Rac1 activity and functionality. Results demonstrated that GAS6/AXL activation significantly upregulated anti-inflammatory cytokines, enhanced efferocytosis, and suppressed pro-inflammatory responses, improving cognitive outcomes. These findings highlight GAS6/AXL as a critical modulator of microglial functions, providing a promising molecular target for treating SAE. GAS6/AXL Pathway Reduces Neuroinflammation in SAE via Regulation of Anti-Inflammatory and Efferocytic Function in M2 Microglia.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were approved by the Animal Ethics Committee of Shanghai Public Health Clinical Center Laboratory Animal Welfare & Ethics Committee (No. 2024-A035-01). All methods were performed in accordance with the relevant guidelines and regulations.
Figures

Similar articles
-
Gas6 Promotes Microglia Efferocytosis and Suppresses Inflammation Through Activating Axl/Rac1 Signaling in Subarachnoid Hemorrhage Mice.Transl Stroke Res. 2023 Dec;14(6):955-969. doi: 10.1007/s12975-022-01099-0. Epub 2022 Nov 3. Transl Stroke Res. 2023. PMID: 36324028
-
Gas6/Axl signaling promotes hematoma resolution and motivates protective microglial responses after intracerebral hemorrhage in mice.Exp Neurol. 2024 Dec;382:114964. doi: 10.1016/j.expneurol.2024.114964. Epub 2024 Sep 15. Exp Neurol. 2024. PMID: 39288830
-
Gas6 induces AIM to suppress acute lung injury in mice by inhibiting NLRP3 inflammasome activation and inducing autophagy.Front Immunol. 2025 Feb 17;16:1523166. doi: 10.3389/fimmu.2025.1523166. eCollection 2025. Front Immunol. 2025. PMID: 40034700 Free PMC article.
-
Central role of microglia in sepsis-associated encephalopathy: From mechanism to therapy.Front Immunol. 2022 Jul 26;13:929316. doi: 10.3389/fimmu.2022.929316. eCollection 2022. Front Immunol. 2022. PMID: 35958583 Free PMC article. Review.
-
Microglial activation and neuroinflammation in acute and chronic cognitive deficits in sepsis.Neuropharmacology. 2025 Apr 1;267:110285. doi: 10.1016/j.neuropharm.2024.110285. Epub 2024 Dec 31. Neuropharmacology. 2025. PMID: 39746541 Review.
References
-
- Hong Y, Chen P, Gao J, Lin Y, Chen L, Shang X. Sepsis-associated encephalopathy: From pathophysiology to clinical management. Int Immunopharmacol. 2023;124:110800. - PubMed
-
- Fülesdi B, Molnar L, Németh N, Molnár C. Sepsis-associated encephalopathy: a review of literature. Neurol India. 2018;66:352. - PubMed
-
- Zhao L, Gao Y, Guo S, Lu X, Yu S, Ge ZZ, et al. Sepsis-associated encephalopathy: insight into injury and pathogenesis. CNSNDDT. 2021;20:112–24. - PubMed
Grants and funding
- No.82272243/National Natural Science Foundation of China (National Science Foundation of China)
- No.82272243/National Natural Science Foundation of China (National Science Foundation of China)
- No.82272243/National Natural Science Foundation of China (National Science Foundation of China)
- No.82272243/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

