Multiscale investigation of collagen structure in human skin and gel matrices using polarization resolved second harmonic generation microscopy
- PMID: 40481016
- PMCID: PMC12144187
- DOI: 10.1038/s41598-025-02536-4
Multiscale investigation of collagen structure in human skin and gel matrices using polarization resolved second harmonic generation microscopy
Abstract
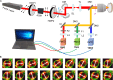
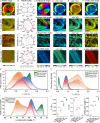
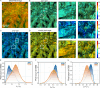
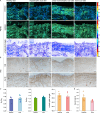
Collagen is critical to the structure and function of skin tissues, with the collagen I/III ratios influencing fibrillogenesis, fiber organization, and skin mechanics. Abnormal collagen organization, such as in fibrosis or scar tissue, compromises both skin functionality and aesthetics. In this study, we employed label-free polarization resolved second harmonic generation (PSHG) microscopy to investigate collagen structure in artificial collagen matrices with various Col I/III ratios at the fibril scale ( 1 to ) and in ex vivo human healthy and scarred skin at the fiber scale ( to ). Complementary third harmonic generation (THG) microscopy provided additional structural information. Our results indicate that an increasing Col I/III ratio is associated with longer fibril length, higher PSHG intensity, and a reduced effective -helix pitch angle of fibrils. In pure Col I, the effective -helix pitch angle is determined to be . These observations indicate alterations in fibril assembly. Furthermore, although the -helix pitch angle of fibers in both healthy and scarred skin was approximately , healthy skin exhibited greater variability in fiber orientation, suggesting a more randomized organization compared to scar tissue. THG imaging further revealed a higher cellular density in scar tissue, consistent with the inflammatory activity associated with wound healing. Immunohistochemical (IHC) staining using dermatansulphate and Col III-specific antibodies confirmed that the Col I/III ratio is higher in healthy skin (2.2) than in scarred skin (1.6). These findings underscore the potential of PSHG microscopy for label-free, quantitative assessment of collagen structure across multiple scales, with THG offering complementary cellular insights. This integrated approach represents a promising strategy for real-time, in vivo monitoring and automated quantification of collagen organization in clinical applications, including dermatology, burn treatment, and fibrosis monitoring.
Keywords: Collagen gel; Collagen type I and type III; Polarization second harmonic generation; Scar skin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Polarization-resolved second harmonic generation imaging of human ovarian cancer.J Biomed Opt. 2018 Jun;23(6):1-8. doi: 10.1117/1.JBO.23.6.066501. J Biomed Opt. 2018. PMID: 29900704 Free PMC article.
-
Differentiation of Col I and Col III isoforms in stromal models of ovarian cancer by analysis of second harmonic generation polarization and emission directionality.Biophys J. 2014 Jan 21;106(2):354-65. doi: 10.1016/j.bpj.2013.10.044. Biophys J. 2014. PMID: 24461010 Free PMC article.
-
Second harmonic generation light quantifies the ratio of type III to total (I + III) collagen in a bundle of collagen fiber.Sci Rep. 2021 Jun 4;11(1):11874. doi: 10.1038/s41598-021-91302-3. Sci Rep. 2021. PMID: 34088955 Free PMC article.
-
Polarization resolved second harmonic microscopy.Methods. 2017 Sep 1;128:105-118. doi: 10.1016/j.ymeth.2017.06.012. Epub 2017 Jun 15. Methods. 2017. PMID: 28624539 Review.
-
Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications.Int J Mol Sci. 2017 Aug 15;18(8):1772. doi: 10.3390/ijms18081772. Int J Mol Sci. 2017. PMID: 28809791 Free PMC article. Review.
References
-
- Miosge, N., Hartmann, M., Maelicke, C. & Herken, R. Expression of collagen type i and type ii in consecutive stages of human osteoarthritis. Histochemistry and cell biology122, 229–236 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

