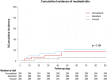
A prospective randomized study that compares three different dressings for the prevention of surgical site infections following major heart surgery
- PMID: 40481025
- PMCID: PMC12144133
- DOI: 10.1038/s41598-025-02533-7
A prospective randomized study that compares three different dressings for the prevention of surgical site infections following major heart surgery
Abstract
Surgical site infection (SSI) is among the most common complication of major heart surgery patients with incidences ranging from 0.5 to 16.5%. Our aim was to compare the incidence, etiology and prognosis of surgical wound infection in three groups of patients with three different type of wound dressing used in a large cohort of patients undergoing cardiac surgery requiring median sternotomy (MHS). This was a randomized, prospective clinical study conducted in 900 adults undergoing MHS at our center from October 10, 2019 to February 22, 2022. Before surgical closure, patients were randomized to 3 different wound dressing groups (300 patients per group): A) conventional gauze (Mepore®), B) Absorbent: polyurethane foam (Mepilex®), or C) Vacuum-negative-pressure therapy (NPWT) wound dressing (PICO®, Smith & Nephew S.A.). Overall, 900 patients were randomized as follows: 300 patients in each group received conventional, absorbent or vacuum wound dressing respectively. Rates of SSI in groups A, B and C were respectively 2.3%, 3% and 3% for superficial SSI (sSSI) (p = 0.848) and 2%, 2% and 0.7% for postsurgical mediastinitis (PSM) (p = 0.313). Mortality in the whole group was 4.4% there being no significant differences between the three groups (4.7%, 5.7% and 3% respectively; p = 0.277). The NPWT system was better at preventing PSM than the other dressings only in the subgroup of patients undergoing coronary artery bypass graft surgery (CABG) with mammary artery grafts. We have not been able to demonstrate significant differences in the incidence of SSI in the whole series with any of the different dressings. The newer, more expensive, NPWT dressing were more effective only at preventing Post-Surgical Mediastinitis in patients undergoing CABG with internal mammary artery grafts. ClinicalTrials.gov identifier (NCT number): NCT03905213.
Keywords: Major heart surgery; Prevention; Surgical wound infection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The Ethics Committee of our institution (Hospital Gregorio Marañon) approved the study (code MICRO.HGUGM.2018–008) and all patients gave their written informed consent before inclusion in this study. Consent was obtained on admission to hospital prior to surgery. Competing interests: The authors declare that they have no conflicts of interest.
Figures
References
-
- Centofanti, P. et al. A prospective study of prevalence of postoperative wound infections after cardiac surger. An updated risk factor analysis. J. Cardiovasc. Surg.48(5), 641–646 (2007). - PubMed
-
- Etchill, E. W. & Whitman, G. J. Commentary: Hospital-acquired infections after cardiac surgery: More dangerous than we may have believed. J. Thorac. & Cardiovasc. Surg.163(6), 2143–2144 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous