Effects of polyunsaturated fatty acids on gastric cancer immunity and immunotherapy
- PMID: 40481119
- PMCID: PMC12144108
- DOI: 10.1038/s41598-025-97644-6
Effects of polyunsaturated fatty acids on gastric cancer immunity and immunotherapy
Abstract
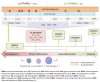
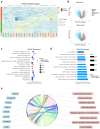
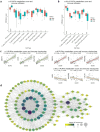
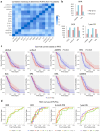
Although immunotherapy has been predominant for advanced gastric cancer treatment, it still has limitations. Polyunsaturated fatty acids (PUFAs) are associated with inflammation while their roles in tumor immune microenvironment (TIME) are unclear. This study explores PUFAs' impacts on gastric cancer immunity and immunotherapy efficacy. Bioinformatics analysis was conducted to identify differential expressions of PUFAs metabolic genes and their immune correlations. Clinical data of advanced gastric cancer patients receiving immunotherapy at Zhongda Hospital (2020-2024), whose serum PUFAs were measured by mass spectrometry (MS), were collected and analyzed. Bioinformatics analysis revealed differential expression of half of PUFAs metabolic genes in gastric cancer. PUFAs metabolism towards Omega-3 (ω-3) tended to increase infiltration of active anti-tumor cells and up-regulate multiple immune checkpoints expressions, while metabolism towards Omega-6 (ω-6) led to opposite TIME outcomes. The clinical study demonstrated that lower serum ω-6/ω-3 ratio, arachidonic acid/eicosapentaenoic acid (AA/EPA) ratio, linoleic acid/alpha-linolenic acid (LA/ALA) ratio and higher EPA were associated with better six-month progression-free survival rate (6-month PFS) and one-year overall survival rate (1-year OS). This study deepens our understandings of TIME in gastric cancer. It clearly demonstrates that maintaining appropriate PUFAs ratios or values is promising in improving prognosis.
Keywords: Bioinformatics analysis; Gastric cancer; Immunotherapy; Mass spectrometry; PUFAs; Tumor immune microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: In the retrospective clinical study, all methods were carried out in accordance with relevant medical research guidelines and regulations. Patients’ blood samples were collected for multiple clinical diagnosis and treatment purposes with the consent of Zhongda Hospital, Southeast University. We confirm that informed consents were obtained from all patients and/or their legal guardians. The study was approved by the Ethics Committee of Zhongda Hospital, Southeast University. Consent to publish: All authors of this study agreed with the publication.
Figures
References
-
- Thrift, A. P., Wenker, T. N. & El-Serag, H. B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol.20(5), 338–349. 10.1038/s41571-023-00747-0 (2023). - PubMed
-
- Catalano, M. et al. Immunotherapy-related biomarkers: Confirmations and uncertainties. Crit. Rev. Oncol. Hematol.192, 104135. 10.1016/j.critrevonc.2023.104135 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

