Deferential nephrotoxicity effect of lanthanum oxide nanoparticle responses to concentration and time in vivo
- PMID: 40481145
- PMCID: PMC12144138
- DOI: 10.1038/s41598-025-04996-0
Deferential nephrotoxicity effect of lanthanum oxide nanoparticle responses to concentration and time in vivo
Abstract
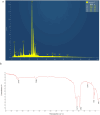
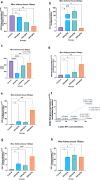
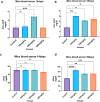
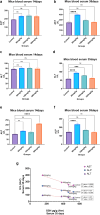
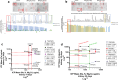
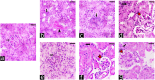
Lanthanum oxide nanoparticles (La₂O₃ NPs) possess unique electronic properties. The increased use of La₂O₃ NPs raises the risk of exposure, potentially leading to bioaccumulation and metabolic disruptions. This study examines the impact of La₂O₃ NPs on the kidneys of mice administered intraperitoneally (i.p) at doses of 60, 150, and 300 mg/kg for 14 and 35 days. Results indicated variations in SOD gene expression and GSH plasma levels that were inversely correlated with NP dosage. A strong positive relationship was found between the inflammatory marker NOS2 transcription and NP dose, confirming pro-inflammatory effects based on concentration. Significant elevations, particularly at lower NP doses, were observed with KIM-1, uric acid, urea, ALP, ALT, and AST, indicating kidney and liver dysfunction. Furthermore, markers of kidney inflammation, as determined by protein array, intensified with prolonged exposure to the lowest concentrations of La₂O₃ NPs. In contrast, higher doses initially caused inflammation that subsided over time. Along with the disruption of mineral balance detected by Inductively Coupled Plasma Mass spectrometry (ICP-MS) (specifically the loss of Ca2+), we believe these factors are the main contributors to nephrotoxicity, the adverse effects of La₂O₃ NPs releasing free La³⁺ ions, which mimic calcium-activating ROS production that worsens at lower concentrations due to their reduced aggregation and enhanced ability to penetrate the cell membrane. However, further studies must confirm whether these effects result from direct nanoparticle circulation in the bloodstream or secondary toxicity mechanisms.
Keywords: Cytokines; ICP-MS; Inflammation; Kidney injury; Lanthanum oxide; Nanoparticle; Protein array; ROS; Rare Earth elements; Toxicity.
© 2025. The Author(s).
Conflict of interest statement
Ethics declarations. Competing interests: The authors declare no competing interests. Ethics approval: The experimental protocol was approved by King Saud University’s Institutional Animal Care and Use Committee (IACUC) (Ref. No.: KSU-SE-22-75).
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

