A multimodal vision foundation model for clinical dermatology
- PMID: 40481209
- PMCID: PMC12353815
- DOI: 10.1038/s41591-025-03747-y
A multimodal vision foundation model for clinical dermatology
Abstract
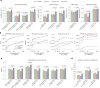
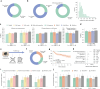
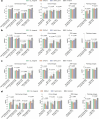
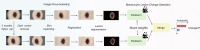
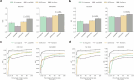
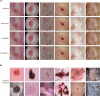
Diagnosing and treating skin diseases require advanced visual skills across domains and the ability to synthesize information from multiple imaging modalities. While current deep learning models excel at specific tasks such as skin cancer diagnosis from dermoscopic images, they struggle to meet the complex, multimodal requirements of clinical practice. Here we introduce PanDerm, a multimodal dermatology foundation model pretrained through self-supervised learning on over 2 million real-world skin disease images from 11 clinical institutions across 4 imaging modalities. We evaluated PanDerm on 28 diverse benchmarks, including skin cancer screening, risk stratification, differential diagnosis of common and rare skin conditions, lesion segmentation, longitudinal monitoring, and metastasis prediction and prognosis. PanDerm achieved state-of-the-art performance across all evaluated tasks, often outperforming existing models when using only 10% of labeled data. We conducted three reader studies to assess PanDerm's potential clinical utility. PanDerm outperformed clinicians by 10.2% in early-stage melanoma detection through longitudinal analysis, improved clinicians' skin cancer diagnostic accuracy by 11% on dermoscopy images and enhanced nondermatologist healthcare providers' differential diagnosis by 16.5% across 128 skin conditions on clinical photographs. These results show PanDerm's potential to improve patient care across diverse clinical scenarios and serve as a model for developing multimodal foundation models in other medical specialties, potentially accelerating the integration of artificial intelligence support in healthcare.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Z.G., V.M., H.P.S., M.J. and P.G. are chief investigators for the Australian Centre of Excellence for Melanoma Imaging and Diagnosis (ACEMID), which was established via an Australian Cancer Research Foundation Major Infrastructure Grant, with research activities supported by NHMRC grants (Cohort Study Grant APP2001517, Centre of Research Excellence Grant APP2044753, Synergy Grant APP2009923) and MRFF Targeted Health System and Community Organisation Research Grant (APP1175082). Z.G. is on the scientific advisory board and a consultant for Optain Health. Although Airdoc has philanthropic donation to the AIM for Health Lab, the company was not involved in any aspect of this research. H.P.S. reported equity in e-derm-consult GmbH and MoleMap NZ Limited, consulting fees from Canfield Scientific Inc and a patent (PCT/AU/2013/000394) licensed to Trajan Medical and Scientific via Uniquest, all outside the submitted work. He is also an executive board member of the International Dermoscopy Society and the Australian Melanoma Clinical Outcome Registry. M.J. holds National Health and Medical Research Council (NHMRC) TRIP Fellowships (APP2006551, APP2009923 and APP2034422). P.T. has received speaker fees from AbbVie and unrestricted educational grants from Lilly. He is an executive board member of the International Dermoscopy Society and past president of the Austrian Society of Dermatopathology. S.S., V.T. and A.B.N. are employees of NVIDIA and own Restricted Stocks. H.K. has received speaker fees from Fotofinder, MSD, Novartis and Pelpharma; license fees from Casio; and equipment from Fotofinder, Casio and Heine. He has served as an advisor for Fotofinder, La Roche-Posay and AI Medical Technology, and is a member of the executive board of the International Dermoscopy Society. P.G. has received honoraria from Metaoptima PTY and travel stipend from L’Oreal. V.M. is supported by an NHMRC Investigator Grant (APP2034976). V.M. has received Victorian Medical Research Acceleration Fund support for the SMARTI Trial with matched contribution from MoleMap; speaker fees from Novartis, Bristol Myers Squibb, Merck and Janssen; and conference travel support from L’Oreal and has participated in advisory boards for MSD, L’Oreal and SkylineDx. V.M. is a board member of the Melanoma and Skin Cancer Trials Group and an advisory member for the Melanoma and Skin Cancer Advocacy Network. The other authors declare no competing interests.
Figures


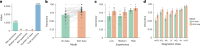



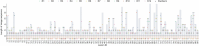
References
-
- Kittler, H., Pehamberger, H., Wolff, K. & Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol.3 3, 159–65 (2002). - PubMed
-
- Primiero, C. A. et al. A narrative review: opportunities and challenges in artificial intelligence skin image analyses using total body photography. J. Invest. Dermatol.144, 1200–1207 (2024). - PubMed
-
- Olsen, C. M. et al. Risk stratification for melanoma: models derived and validated in a purpose-designed prospective cohort. J. Natl Cancer Inst.110, 1075–1083 (2018). - PubMed
-
- Usher-Smith, J. A., Emery, J., Kassianos, A. P. & Walter, F. M. Risk prediction models for melanoma: a systematic review. Cancer Epidemiol. Biomark. Prev.23, 1450–1463 (2014). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

