Adherence to STARD guidelines in miRNA diagnostic accuracy studies for hepatocellular carcinoma: a systematic review
- PMID: 40481407
- PMCID: PMC12142931
- DOI: 10.1186/s12874-025-02610-5
Adherence to STARD guidelines in miRNA diagnostic accuracy studies for hepatocellular carcinoma: a systematic review
Abstract
Background: Accumulating evidence demonstrates that miRNAs exhibit enhanced diagnostic sensitivity and specificity compared to the conventional hepatocellular carcinoma (HCC) biomarker alpha-fetoprotein (AFP), particularly showing promising clinical utility in early-stage HCC detection. However, insufficient transparency in methodology reporting compromises the validity assessment of these findings and hinders their translational applications. In this study, we investigated the adherence to the STARD criteria in studies investigating the diagnostic accuracy of miRNA for liver cancer. In addition, the quality of reporting was examined to identify factors influencing the quality of reporting.
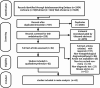
Method: A comprehensive search strategy was performed on the PubMed, EMBASE, Cochrane Library, and Web of Science, as of March 30, 2023. Clinical trials investigating the diagnostic value of miRNA for HCC diagnosis were included in the analysis. The eligible studies were checked against adherence to the STARD criteria. Factors determining quality of studies were evaluated through subgroup analyses. All statistical analyses were performed using SPSS (varsion25.0).
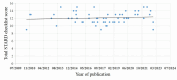
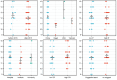
Results: Sixty-two eligible studies, all published between 2010 and 2022, were eventually included in the final analysis. The results revealed a moderate overall adherence to STARD 2015, with an average of 12.1 (52.6%) of the 23 items reported. Subgroup analysis revealed that adherence to the STARD 2015 varied among countries (USA 58.7%;Egypt 46.5%)(p<0.05).
Conclusion: The quality of studies reporting the diagnostic accuracy of miRNAs in HCC was average and did not increase after the publication of STARD 2015. Our findings rise awareness regarding the need to improve STARD standards, implying that more journals need to include STARD standards for relevant manuscripts. In addition, editorial and peer approval procedures should adopt measures that will aim to improve reporting quality.
Keywords: Checklist; Diagnosis; HCC; MiRNA; Report; STARD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: The authors agreed to publish. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2021 Apr 15;4(4):CD013346. doi: 10.1002/14651858.CD013346.pub2. Cochrane Database Syst Rev. 2021. PMID: 33855699 Free PMC article.
-
Assessment of Standards for Reporting of Diagnostic Accuracy (STARD) 2015 guideline adherence in medical imaging diagnostic accuracy studies published in 2023.J Clin Epidemiol. 2025 Mar;179:111654. doi: 10.1016/j.jclinepi.2024.111654. Epub 2024 Dec 27. J Clin Epidemiol. 2025. PMID: 39733974
-
Serum miRNA-101 expression signature as non-invasive diagnostic biomarker for Hepatitis C virus-associated hepatocellular carcinoma in Egyptian patients.Sci Rep. 2025 Jan 3;15(1):645. doi: 10.1038/s41598-024-81207-2. Sci Rep. 2025. PMID: 39753619 Free PMC article.
-
Comprehensive evaluation of microRNA as a biomarker for the diagnosis of hepatocellular carcinoma.World J Gastroenterol. 2022 Aug 7;28(29):3917-3933. doi: 10.3748/wjg.v28.i29.3917. World J Gastroenterol. 2022. PMID: 36157551 Free PMC article.
-
Diagnostic Performance of microRNA-122 and microRNA-224 in Hepatitis C Virus-Induced Hepatocellular Carcinoma (HCC).Asian Pac J Cancer Prev. 2019 Aug 1;20(8):2515-2522. doi: 10.31557/APJCP.2019.20.8.2515. Asian Pac J Cancer Prev. 2019. PMID: 31450927 Free PMC article.
References
-
- Shlobin NA, et al. Reporting policies in neurosurgical journals: A Meta-Science study of the current state and case for standardization. World Neurosurg. 2022;158:11–23. - PubMed
-
- White SJ. Systematic review of adherence to the standards for reporting of diagnostic accuracy studies (STARD) 2015 reporting guideline in cerebral aneurysm imaging diagnostic accuracy studies. J Clin Neurosci. 2023;115:89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 24JRRA431/Natural Science Foundation of Gansu Province
- 82272405/National Natural Science Foundation of China
- 23JRRA1503/General Projects of Gansu Provincial Joint Research Fund
- GSWSQN2022-02/Health Industrial Outstanding Youth Talent Project of Gansu Province
- 25YFFA052/Key Research and Development Program of Gansu Province
LinkOut - more resources
Full Text Sources
Medical