Automatic target-seeking nanoparticle inhibiting orthotopic drug-resistant colon cancer and liver metastases via regulating cancer cell adhesion and proliferation
- PMID: 40481460
- PMCID: PMC12142993
- DOI: 10.1186/s12951-025-03422-x
Automatic target-seeking nanoparticle inhibiting orthotopic drug-resistant colon cancer and liver metastases via regulating cancer cell adhesion and proliferation
Abstract
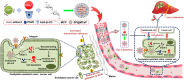
Galectin-3 (Gal-3) plays an important role in adhesion and proliferation of cancer cells. The level of Gal-3 in blood and the expression of Gal-3 in colon cancer tissue are significantly increased in patient with colon cancer. The elevated Gal-3 promotes the migration and drug resistance of colon cancer. Therefore, Gal-3 is a promising target to inhibit the growth and metastases of cancer cells. Besides, integrin αvβ3, a receptor of Gal-3, is highly expressed in colon cancer cell and blood vessel in colon cancer tissue. In this paper, an automatic target-seeking nanoparticle (SP@MCaP) contained siGal-3 and paris saponin VII (PSVII) was prepared. In vivo, by automatically capturing Gal-3 in the blood circulation, SP@MCaP actively recognized cancer tissue vessel and drug-resistant colon cancer cells with elevated integrin αvβ3 expression, resulting in specifical accumulation in orthotopic drug-resistant colon cancer tissue. SP@MCaP diminished Gal-3 level in serum and orthotopic drug-resistant colon cancer tissue, and then suppressed the proliferation of drug-resistant colon cancer cells. Importantly, SP@MCaP reconstructed the adhesion of drug-resistant colon cancer cells and reversed the immunosuppressive microenvironment in orthotopic drug-resistant colon cancer tissue and liver tissue. Finally, under the synergistic effect of siGal-3 and PSVII, SP@MCaP successfully inhibited the growth of orthotopic drug-resistant colon cancer and its liver metastases. In a word, this paper explored a novel concept of the active co-delivery of siGal-3 and PSVII by modification of nanoparticle, which holds promise for targeted therapy in orthotopic drug-resistant colon cancer and its liver metastases.
Keywords: Cell adhesion; Drug-resistant colon cancer; Galectin-3; Integrin αvβ3; Paris saponin VII.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Air Force Medical University Institutional Animal Care and Utilization Committee (No: IACUC-20220812). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures









References
-
- Zhou J, Xu B, Shen Q, Zhang Z, Hu Y, Wang M, et al. Identification and biological evaluation of fused tetrahydroisoquinoline derivatives as Wnt/β-catenin signaling inhibitors to suppress colorectal cancer. Eur J Med Chem. 2024;276:116664. - PubMed
-
- Yadav R, Bhawale R, Srivastava V, Pardhi E, Bhalerao HA, Sonti R, et al. Innovative nanoparticulate strategies in colon cancer treatment: a paradigm shift. AAPS PharmSciTech. 2024;25(3):52. - PubMed
MeSH terms
Substances
Grants and funding
- 82073775/National Natural Science Foundation of China
- 2023-ZDYJSY-001/Key Laboratory of New Drug Delivery System and New Technology for Formulation, Shaanxi Administration of Traditional Chinese Medicine
- 2023JSYX16/Research Project from Air Force Medical University
- 2023-YBSF-221/Science and Technology Research and Development Program of Shaanxi Province
LinkOut - more resources
Full Text Sources
Medical

