Identification and heterologous expression of an NRPS biosynthetic gene cluster responsible for the production of the pyrazinones Ichizinone A, B and C
- PMID: 40481494
- PMCID: PMC12144821
- DOI: 10.1186/s12934-025-02753-6
Identification and heterologous expression of an NRPS biosynthetic gene cluster responsible for the production of the pyrazinones Ichizinone A, B and C
Abstract
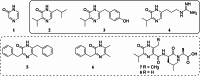
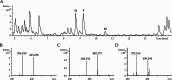
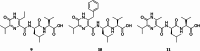
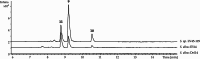
Pyrazinones are a growing family of microbial NRPS-derived natural products showing interesting biological activities. These compounds are characterized by the presence of either a di- or trisubstituted heterocyclic, nonaromatic 2(1 H)-pyrazinone core in their structure. The most commonly occurring disubstituted pyrazinone natural products are synthesized through a dipeptide intermediate, which is further cyclized to yield the pyrazinone moiety. Trisubstituted pyrazinones are seldom found in natural products, with JBIR56 and JBIR57, isolated from marine Streptomyces, being notable examples. In contrast to the simply organized disubstituted pyrazinones, JBIR56 and JBIR57 are syn-thesized as tetrapeptides with unnatural beta-amino acid residue involved in the for-mation of the pyrazinone moiety. Despite interesting structural features, biosynthetic routes leading to the production of these compounds have not been reported yet. Here we report the discovery of new members of trisubstituted pyrazinone family- tetrapeptides ichizinones A-C in Streptomyces sp. LV45-129. Through sequence analysis and heterologous expression, a biosynthetic gene cluster encoding ichizinone production was identified. Based on gene annotation and sequence homology, a biosynthetic model was suggested. The presented results provide insights into the biosynthesis of rare trisubstituted pyrazinone natural products.
Keywords: Streptomyces; Actinobacteria; Biosynthesis; Gene cluster; Heterologous expression; NRPS; Pyrazinone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Zimmermann M, Fischbach MA. A family of Pyrazinone natural products from a conserved nonribosomal peptide synthetase in Staphylococcus aureus. Chem Biol. 2010;17:925–30. - PubMed
-
- Tatsuta K, Tsuchiya T, Someno T, Umezawa S, Umezawa H. Arglecin, a new microbial metabolite isolation and chemical structure. J Antibiot (Tokyo). 1971;24:735–46. - PubMed
-
- Tatsuta K, Fujimoto K, Yamashita M, Tsuchiya T, Umezawa S. Argvalin, a new microbial metabolite: isolation and structure. J Antibiot (Tokyo). 1973;26:606–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

