Nutrient-gene therapy as a strategy to enhance CAR T cell function and overcome barriers in the tumor microenvironment
- PMID: 40481543
- PMCID: PMC12144745
- DOI: 10.1186/s12967-025-06606-z
Nutrient-gene therapy as a strategy to enhance CAR T cell function and overcome barriers in the tumor microenvironment
Abstract
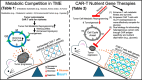
Cancer immunotherapy is transforming the treatment landscape of both hematological and solid cancers. Although T-cell-based adoptive cell transfer (ACT) therapies have demonstrated initial success, several recurrent obstacles limit their long-term anti-tumor efficacy, including: (1) lack of antigen specificity; (2) poor long-term survival of transplanted T cells in vivo; and (3) a hostile tumor microenvironment (TME). While numerous approaches have been explored to enhance the antigen specificity of Chimeric Antigen Receptor (CAR) T-cell therapies, the field still lacks an effective strategy to optimize the long-term retention and in vivo expansion of engrafted T cells within the TME-a critical factor for the durable efficacy of T-cell-based immunotherapies for both blood and solid cancers. Here, we hypothesize that the success of CAR T-cell therapy can be enhanced by targeting donor T cells' ability to compete with cancer cells for key nutrients, thereby overcoming T-cell exhaustion and sustaining durable anti-tumor function in the TME. To explore this hypothesis, we first provide a comprehensively review of the current understanding of the metabolic interactions (e.g., glucose metabolism) between T cells and tumor cells. To address the challenges, we propose an innovative strategy: utilizing nutrient gene therapy (genetic overexpression of glucose transporter 1, GLUT1) to fortify the metabolic competency of adoptive CAR T-cells, deprive tumors of critical metabolites and ATP, and disrupt the TME. Altogether, our proposed approach combining precision medicine (adoptive CAR T-cell therapy) with tumor metabolism-targeting strategies offers a promising and cost-effective solution to enhance the efficacy and durability of ACT therapies, ultimately improving outcomes for cancer patients.
Keywords: Adoptive cell therapy; CAR T; Cancer immunotherapy; GLUT1; Gene therapy; Glucose; Metabolite; Nutrient; TME; Warburg effect.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors agree to publish. Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
CAR-T therapy in solid tumors.Cancer Cell. 2025 Apr 14;43(4):665-679. doi: 10.1016/j.ccell.2025.03.019. Cancer Cell. 2025. PMID: 40233718 Review.
-
Optimizing CAR-T cell function in solid tumor microenvironment: insights from culture media additives.Curr Res Transl Med. 2025 Apr-Jun;73(2):103491. doi: 10.1016/j.retram.2024.103491. Epub 2024 Dec 31. Curr Res Transl Med. 2025. PMID: 39798497 Review.
-
IDO1 inhibition enhances CLDN18.2-CAR-T cell therapy in gastrointestinal cancers by overcoming kynurenine-mediated metabolic suppression in the tumor microenvironment.J Transl Med. 2025 Mar 5;23(1):275. doi: 10.1186/s12967-025-06276-x. J Transl Med. 2025. PMID: 40045363 Free PMC article.
-
Cell metabolism-based optimization strategy of CAR-T cell function in cancer therapy.Front Immunol. 2023 Jun 5;14:1186383. doi: 10.3389/fimmu.2023.1186383. eCollection 2023. Front Immunol. 2023. PMID: 37342333 Free PMC article. Review.
-
Engineering CAR-T Therapeutics for Enhanced Solid Tumor Targeting.Adv Mater. 2025 Jun;37(23):e2414882. doi: 10.1002/adma.202414882. Epub 2025 Jan 2. Adv Mater. 2025. PMID: 39745117 Free PMC article. Review.
References
-
- Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity. 2023;56(10):2188–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

