Genetic deletion of microsomal prostaglandin E synthase-1 promotes imiquimod-induced psoriasis in mice
- PMID: 40481552
- PMCID: PMC12142878
- DOI: 10.1186/s41232-025-00385-2
Genetic deletion of microsomal prostaglandin E synthase-1 promotes imiquimod-induced psoriasis in mice
Abstract
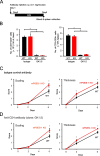
Background: Psoriasis is a chronic inflammatory disease associated with abnormalities in the immune system. Microsomal prostaglandin E synthase-1 (mPGES-1), a terminal enzyme for prostaglandin (PG) E2 biosynthesis, is highly expressed in the skin of psoriasis patients. However, the detailed role of mPGES-1 in psoriasis remains unclear. In the present study, we aimed to investigate the role of mPGES-1 in psoriasis-like skin inflammation induced by imiquimod (IMQ), a well-established model of psoriasis.
Methods: Psoriasis was induced in mPGES-1-deficient (mPGES-1-/-) and wild-type (WT) mice by administering IMQ for 6 days. Psoriasis was evaluated based on the scores of the macroscopic symptoms, including skin scaling, thickness, and redness, and on the histological features. The skin expression of mPGES-1 was determined by real-time polymerase chain reaction and Western blotting. The impact of mPGES-1 deficiency on T-cell immunity was determined by flow cytometry and γδ T-cell depletion in vivo with anti-T-cell receptor (TCR) γδ antibody.
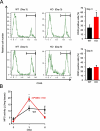
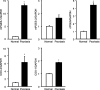
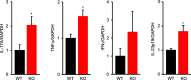
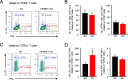
Results: The inflamed skin of mPGES-1-/- mice showed severe symptoms after the administration of IMQ. Histological analysis further showed significant exacerbation of psoriasis in mPGES-1-/- mice. In WT mice, the mPGES-1 expression was highly induced at both mRNA and protein levels in the skin, and PGE2 increased significantly after IMQ administration, while the PGE2 production was largely abolished in mPGES-1-/- mice. These data indicate that mPGES-1 is the main enzyme responsible for PGE2 production in the skin. Furthermore, the lack of mPGES-1 increased the numbers of IL-17A-producing γδ T cells in the skin with IMQ-induced psoriasis, and γδ T-cell depletion resulted in a reduction of the facilitated psoriasis symptoms under the condition of mPGES-1 deficiency.
Conclusions: Our study results demonstrate that mPGES-1 is the main enzyme responsible for skin PGE2 production, and that mPGES-1 deficiency facilitates the development of psoriasis by affecting the development of T-cell-mediated immunity. Therefore, mPGES-1 might impact both skin inflammation and T-cell-mediated immunity associated with psoriasis.
Keywords: Cyclooxygenase; Immunity; Inflammation; Interleukin-17 A; Prostaglandin E synthase; Prostaglandin E2; Psoriasis; γδ T cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Animal Research and Ethics Committee of Kitasato University (approval number: Ei-ken 24–06), and all experiments involving the mPGES-1−/− mice were approved by the Safety Committee for Recombinant DNA Experiments of Kitasato University (approval number: 4929). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures






References
Grants and funding
- 21K06603/JSPS KAKENHI
- Student Research Program 2022-2023/Kitasato University Graduate School of Medical Sciences
- Research Program 2024/Regenerative Medicine and Cell Design Research Facility
- Research Project 2021-1017/Kitasato University School of Allied Health Sciences
- Research Project 2022-1008/School of Allied Health Sciences, Kitasato University
LinkOut - more resources
Full Text Sources

