Effect of adding fenofibrate versus curcumin to glimepiride in patients with type 2 diabetes: a randomized controlled trial
- PMID: 40481568
- PMCID: PMC12144694
- DOI: 10.1186/s40360-025-00950-y
Effect of adding fenofibrate versus curcumin to glimepiride in patients with type 2 diabetes: a randomized controlled trial
Abstract
Background: Type 2 diabetes is a recognized risk factor for the development of cardiovascular disease. Fenofibrate and curcumin were found to be effective in improving hyperlipidemia in patients with diabetes. This study aimed to evaluate the effect of adding fenofibrate versus curcumin on weight, glycemic status, lipids profile, high-sensitivity C-reactive protein (hs-CRP), fetuin-A, and sirtuin 1 in patients with type 2 diabetes treated with glimepiride.
Method: In a double-blind, randomized control trial, 60 patients with type 2 diabetes mellitus were randomly assigned into three groups: Group I was given placebo; Group II curcumin 1100 mg; Group III fenofibrate 160 mg (each administered orally once daily) to ongoing glimepiride 4 mg therapy administered orally once daily for three months. Inclusion criteria were as follows: patients aged 35-70 years, those with uncontrolled type 2 diabetes, hyperlipidemia, and those treated with glimepiride 4 mg. Exclusion criteria were as follows: other types of diabetes, pregnancy, abnormal liver or kidney function tests, using other anti-diabetes medications, and non-adherence to medications. At baseline and after three months of intervention, anthropometric measurements were measured, and blood samples were collected for biochemical analysis of blood glucose, glycated hemoglobin (HbA1c), lipid profile, hs-CRP, fetuin-A, and sirtuin 1. Paired t-test and one-way ANOVA were used for normally distributed data. However, the Wilcoxon signed-rank and the Kruskal-Wallis tests were used to analyze non-normally distributed data.
Results: Three months after the intervention, when the three groups were compared, no significant differences were found regarding weight, body mass index, fasting blood glucose, two-hour postprandial glucose (2 h-PPG), and HbA1c (p > 0.05). Compared to placebo, significant decreases were observed in total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), very-low-density lipoprotein cholesterol (VLDL-C), coronary risk index (CRI), atherogenic index (AI), and hs-CRP and increasing sirtuin 1 in the fenofibrate (p < 0.001) and curcumin (p < 0.05) groups. High-density lipoprotein cholesterol (HDL-C) levels in the fenofibrate group were found to be significantly higher than in the placebo group (p < 0.001). Furthermore, when compared to curcumin, fenofibrate significantly reduced waist circumferences and fetuin-A and increased sirtuin 1 (p < 0.05).
Conclusion: Both fenofibrate and curcumin are effective at decreasing lipid profiles and improving inflammatory markers in patients with type 2 diabetes. Fenofibrate might have a superior effect in reducing waist circumference, decreasing fetuin-A, and increasing sirtuin 1.
Trial registration: This trial was registered (August 27, 2020) on clinical trial.gov with an identification code NCT04528212. https://www.
Clinicaltrials: gov/study/NCT04528212 .
Keywords: Curcumin; Fenofibrate; Fetuin-A; Hs-CRP; Sirtuin 1; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval for the trial was obtained from the Research Ethics Committee, Faculty of pharmacy, Damanhour University, Damanhour, Egypt (with code no.420PP24). Furthermore, this study has been performed following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was taken from the patients before the study. Consent for publication: Not applicable. Competing interests: No conflict of interest was reported by the authors.
Figures
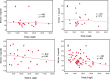
Similar articles
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD009966. doi: 10.1002/14651858.CD009966.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jul 30;8:CD009966. doi: 10.1002/14651858.CD009966.pub3. PMID: 28238223 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium 2021.
-
- Einarson TR, Acs A, Ludwig C, Panton UH.Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21(7):881–90. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

