Results of a phase 1/2 study of sacituzumab tirumotecan in patients with unresectable locally advanced or metastatic solid tumors refractory to standard therapies
- PMID: 40481574
- PMCID: PMC12142944
- DOI: 10.1186/s13045-025-01705-2
Results of a phase 1/2 study of sacituzumab tirumotecan in patients with unresectable locally advanced or metastatic solid tumors refractory to standard therapies
Abstract
Background: Sacituzumab tirumotecan (sac-TMT) is an antibody-drug conjugate composed of an anti-TROP2 monoclonal antibody coupled to a cytotoxic belotecan-derived topoisomerase I inhibitor (KL610023) via a novel linker. We report results from the phase 1 dose-escalation cohorts in advanced solid tumors and phase 2 expansion cohorts for metastatic triple-negative breast cancer (TNBC) from the first-in-human MK-2870-001 (KL264-01) study (NCT04152499).
Methods: Patients had unresectable locally advanced/metastatic solid tumors refractory to standard therapies. In the phase 1 dose-escalation cohorts, patients had unresectable locally advanced/metastatic solid tumors refractory to standard therapies. Sac-TMT was administered by intravenous administration every 2 weeks at 2 to 12 mg/kg. In phase 2, patients with TNBC and HR+/HER2- breast cancer received sac-TMT per recommended doses for expansion (RDEs) identified in phase 1. Primary objectives were determining maximum tolerated dose (MTD) of sac-TMT and establishing RDEs (phase 1) and determining ORR per RECIST v1.1 by investigator assessment (phase 2). Adverse events were assessed per NCI-CTCAE version 5.0.
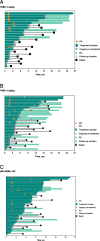
Results: Thirty patients were enrolled in phase 1 and received sac-TMT 2 mg/kg (n = 4), 4 mg/kg (n = 7), 5 mg/kg (n = 7), 5.5 mg/kg (n = 5), and 6 mg/kg (n = 7). Five patients had dose-limiting toxicities: grade 3 stomatitis at 4, 5.5, and 6 mg/kg; grade 3 rash at 5 mg/kg; and grade 3 urticaria at 6 mg/kg. MTD was 5.5 mg/kg and RDEs were 4 and 5 mg/kg. In the phase 2 dose expansion, ORR (95% CI) was 34.8% (16.4%, 57.3%) in the 4-mg/kg group (n = 23) and 38.9% (23.1%, 56.5%) in the 5-mg/kg group (n = 36) for TNBC. ORR (95% CI) was 31.7% (18.1%, 48.1%) for HR+/HER2- breast cancer (n = 41).
Conclusions: Sac-TMT demonstrated manageable safety profile in patients with unresectable locally advanced/metastatic solid tumors and promising antitumor activity in metastatic TNBC and HR+/HER2 - breast cancer. Sac-TMT is being investigated in phase 3 studies.
Trial registration: ClinicalTrials.gov, NCT04152499.
Keywords: Antibody–drug conjugate; HR+/HER2− breast cancer; Sac-TMT; Triple-negative breast cancer.
© 2025. Merck & Co., Inc., Rahway, NJ, USA and its affiliates, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd., Chengdu, China, and the Authors.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol and its amendments were approved by an institutional review board or institutional ethics committee at each study site, including the University of Texas MD Anderson Cancer Center Institutional Review Board (IRB 1 IRB00000121) and the Medical Ethics Committee of Shanghai East Hospital Affiliated to Tongii University School of Medicine (2020 [015]). Competing interests: Quchang Ouyang: None declared. Jordi Rodon: Dr Rodon reports non-financial support and reasonable reimbursement for travel from European Society for Medical Oncology, American Society of Medical Oncology, National Taiwan University Cancer Center, 280-Biotech, Dava Oncology, STOP Cancer; receiving consulting and travel fees from Ellipses Pharma, IONCTURA, Sardona, Mekanistic, Amgen, Merus, MonteRosa, Aadi, and Bridgebio (including serving on the scientific advisory board); Consulting fees from Vall d'Hebron Institute of Oncology, Chinese University of Hong Kong, Boxer Capital, LLC, Tang Advisors, LLC, Guidepoint, and Axiom; receiving research funding from Blueprint Medicines, MSD, Hummingbird, AstraZenneca, 280 Bio, Vall d'Hebron Institute of Oncology/Cancer Core Europe; and serving as investigator in clinical trials with Cancer Core Europe, Symphogen, BioAlta, Pfizer, Kelun-Biotech, GlaxoSmithKline, Taiho, Roche Pharmaceuticals, Hummingbird, Yingli, Bicycle Therapeutics, Merus, AadiBioscience, ForeBio, Loxo Oncology, Hutchinson MediPharma, Ideaya, Amgen, Tango Therapeutics, Mirati, Linnaeus Therapeutics, MonteRosa, Kinnate, Yingli, Debio, BioTheryX, Storm Therapeutics, Beigene, MapKure, Relay, Novartis, FusionPharma, C4 Therapeutics, Scorpion Therapeutics, Incyte, Fog Pharmaceuticals, Tyra, Nuvectis Pharma, Hotspot Pharma, Adcentrix, Vividion, AstraZenneca, Alnylam, Immuneering Corp, Alterome, Exelixis. Yan Liang: None declared. Xinhong Wu: None declared. Qun Li: None declared. Lihua Song: None declared. Min Yan: None declared. Zhongsheng Tong: None declared. YunPeng Liu: None declared. Zev A. Wainberg: None declared. Ying Wang: None declared. Cuizhi Geng: None declared. Susanna V. Ulahannan: Research funding to institution: AbbVie Inc, Adlai Nortye, ArQule Inc, AstraZeneca, Atreca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Ciclomed LLC, Erasca, Evelo Biosciences Inc., Exelexis, G1 Therapeutics Inc, GlaxoSmithKline GSK, IGM biosciences, Incyte, Isofol, Klus Pharma Inc., Macrogenics, MSD, Mersana Therapeutics, OncoMed Pharmaceuticals Inc., Pfizer, Regeneron Inc., Revolution Medicines Inc., Synermore Biologics Co, Takeda, Tarveda Therapeutics, Tesaro, Tempest, and Vigeo Therapeutics Inc.; Advisory Board for Eisai, AstraZeneca, and IgM biosciences. Guohua Yu: None declared. Manish R. Sharma: Research funding to institution: Arcus Bioscience, AbbVie, Adcentrx Therapeutics, Alkermes, ALX Oncology, Artios Pharma, Astellas, Aulos Bioscience, BioNTech, Black Diamond Therepeutics, Boundless Bio, Bolt Biotherapeutics, Boehringer Ingelheim, Bright Peak Therapeutics, Bristol Myers Squibb, Biontech, Celgene, Chugai Pharmaceutical, Circle Pharma, Cullinan, Compugen, Constellation Pharmaceuticals, Debiopharm, Deciphera Pharmaceuticals, eFFECTOR, Elevation Oncology, Exelixis, GenMab, Gilead Sciences, GlaxoSmithKline, Iambic Therapeutics, IgM Biosciences, Ikena, Inhibrx, Incyte, Janssen, KLUS Pharma, Lilly, LOXO Oncology, Macrogenics, MSD, Mirati, NGM Biosciences, Onconova, Opna Bio, Palleon, Pfizer, Pliant Therapeutics, ProfoundBio, PureTech, Qualigen, Regeneron Pharmaceuticals, Repare Therapeutics, Revolution Medicines, Roche, Sapience Therapeutics, Seagen, Shattuck Labs, Stemline Therapeutics, Symphogen, Tempest Therapeutics, Theratechnologies, Tizona Therapeutics, Volastra Therapeutics, and 23andMe Therapeutics; Consulting for Pliant Therapeutics, Enlaza Therapeutics, and Aadi Bioscience; Stock Ownership of AbbVie, Amgen, Bristol Myers Squibb, Gilead Sciences, Johnson & Johnson, Moderna, Regeneron, and West Pharmaceutical. Xiang Wang: None declared. Judy S. Wang: Research funding by Kelun/Klus and MSD, to institution only. Alexander Spira: Research funding from Abbvie, ADC Therapeutics, Alkermes, Amgen, Arch Therapeutics, ArriVent Biopharma, Astellas Pharma, Astex Pharmaceuticals, AstraZeneca, Black Diamond Therapeutics, Blueprint Medicines, BluPrint Oncology, Boehringer Ingelheim, Bristol-Myers Squibb, CytomX Therapeutics, Daiichi Sankyo, Gritstone Bio, Ignyta, Incyte, Janssen Oncology, LAM Therapeutics, Loxo, Macrogenics, Medikine, MedImmune, mersana, Mirati Therapeutics, Nalo Therapeutics, Novartis, Plexxikon, Regeneron, Revolution Medicines, Revolution Medicines, Roche, Rubius Therapeutics, Scorpion Therapeutics, Synthekine, and Takeda; Consulting or advisory role for Amgen, Array BioPharma, AstraZeneca/MedImmune, AstraZeneca/MedImmune, Black Diamond Therapeutics, Blueprint Medicines, Bristol-Myers Squibb, Daiichi Sankyo/Astra Zeneca, Gritstone Bio, Incyte, Janssen Research & Development, Jazz Pharmaceuticals, Lilly, MSD, Mersana, Mirati Therapeutics, Novartis, Regeneron, Sanofi, and Takeda; Honoraria from Amgen, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, CytomX Therapeutics, Janssen Oncology, MSD, Novartis, and Takeda; Stock and Other Ownership Interests for Lilly; and Leadership role for Next Oncology. Weihong Zhao: None declared. Rachel E. Sanborn: Advisor for Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, G1 Therapeutics, GE HealthCare, Gilead, Janssen Oncology, Johnson & Johnson, Inhibrx, Lilly Oncology, Regeneron, and Sanofi Aventis; Honoraria for educational presentations from Illumina, OncLive, and PRIME Education; Steering Committee for AstraZeneca, BeiGene, GlaxoSmithKline, Janssen Oncology, and Johnson & Johnson; Honoraria from CurioScience and IDEOlogy. Ying Cheng: None declared. Xian Wang: None declared. Gesha Liu: None declared. Yaling Li: None declared. Junyou Ge: None declared. Elliot Chartash: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. Omobolaji O. Akala: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. Yongmei Yin: None declared.
Figures


References
-
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 4.2023 https://www.nccn.org/. Accessed February 15, 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

