Pharmacokinetics and Pharmacodynamics of Intranasal Diacetylmorphine in Heroin-Assisted Treatment for Severe Opioid Use Disorder
- PMID: 40481986
- PMCID: PMC12263744
- DOI: 10.1007/s40263-025-01189-1
Pharmacokinetics and Pharmacodynamics of Intranasal Diacetylmorphine in Heroin-Assisted Treatment for Severe Opioid Use Disorder
Abstract
Background: Intranasal diacetylmorphine (IN DAM) represents a promising new route of administration, which is currently under investigation as a novel treatment approach for opioid use disorder in Switzerland. This study characterized the pharmacokinetics and pharmacodynamics of therapeutically relevant intranasal doses of DAM and its metabolites in patients with severe opioid use disorder.
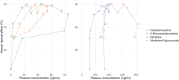
Methods: In this prospective observational study, patients on intranasal heroin-assisted treatment (HAT) in Basel, Switzerland, self-administered their usual dose of IN DAM before receiving their daily maintenance dose. Inclusion criteria were age ≥ 18 years and current participation in IN HAT. Plasma concentrations of diacetylmorphine, 6-monoacetylmorphine (6-MAM), morphine, morphine-6-glucuronide, and morphine-3-glucuronide were measured using liquid chromatography-tandem mass spectrometry at baseline and at 2, 5, 10, 15, 20, 30, 40, 50, 60, 120, and 180 min. Acute subjective effects and cravings were assessed repeatedly using visual analog scales, withdrawal symptoms were measured using the Short Opiate Withdrawal Scale, and autonomic responses were recorded over the 3 h after IN DAM administration.
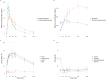
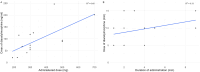
Results: In total, 14 patients were included in the study. The mean self-administered IN DAM dose was 346 mg (range 190-700 mg) delivered over a mean time of 3.8 min (range 1-9 min). IN DAM elicited moderate-to-strong peak drug effects, with marked reductions in heroin craving and withdrawal symptoms. No clinically relevant respiratory depression or decrease in oxygenation saturation was observed. Subjective effects occurred rapidly within the first 2 min after the start of administration. These effects gradually increased and peaked at 30-35 min, which paralleled the rising plasma concentrations of DAM and 6-MAM, while the sustained heroin-like effects best correlated with morphine and morphine-6-glucuronide plasma concentrations. Plasma half-lives of diacetylmorphine and 6-monoacetylmorphine were longer, and time to maximal plasma concentration was later than previously reported, suggesting saturated absorption at high intranasal doses and volumes.
Conclusions: IN DAM has a good safety profile and should be considered as an effective alternative for patients in HAT, offering rapid onset of effects without significant side effects. Optimization of intranasal delivery may further improve absorption and clinical utility.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: The Ethics Committee for Northwest and Central Switzerland (project ID: 2020-00354) approved the study, which was performed in accordance with the Sixth Revision of the Declaration of Helsinki. Funding: This study was funded by the Swiss Federal Office of Public Health. The funding covered costs related to laboratory analyses, equipment and participant compensation. The funding source had no role in the design of the study, data collection, analysis, interpretation of the data, or the writing of the manuscript. Open access funding was provided by the University of Basel as part of an institutional agreement with Springer Nature. Conflicts of Interest: The authors declare they have no conflict of interest. Consent to Participate: Written informed consent was obtained from all individual participants included in the study. Consent to Publish: Not applicable. Data Availability: The data that support the findings are available from the corresponding author upon reasonable request. Code Availability: Not applicable. Author Contributions: A.G. and M.V. conceived the study. A.G., S.B.V., M.M., and B.K. carried out the clinical procedures and blood sampling. S.B.V., J.T., D.L., and U.D. were responsible for pharmacokinetic analyses. M.V., K.M.D., and M.E.L. supervised the project. M.V. was responsible for funding acquisition. A.G., J.N.W., and S.B.V. wrote the initial manuscript draft. All authors were involved in revising the manuscript. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Figures

References
-
- Vogel M, Meyer M, Westenberg JN, Kormann A, Simon O, Salim Hassan Fadlelseed R, et al. Safety and feasibility of intranasal heroin-assisted treatment: 4-week preliminary findings from a Swiss multicentre observational study. Harm Reduct J. 2023;20:1–8. 10.1186/S12954-023-00731-Y/TABLES/4. - DOI - PMC - PubMed
-
- Westenberg JN, Meyer M, Strasser J, Krausz M, Dürsteler KM, Falcato L, et al. Feasibility, safety, and acceptability of intranasal heroin-assisted treatment in Switzerland: protocol for a prospective multicentre observational cohort study. Addict Sci Clin Pract. 2023;18:1–8. 10.1186/s13722-023-00367-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

