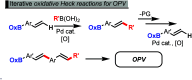
Modular Assembly of Oligo(phenylenevinylene)s via Iterative Linear-Selective Oxidative Heck Reactions with Protected Boronic Acids
- PMID: 40482051
- PMCID: PMC12186689
- DOI: 10.1021/acs.orglett.5c01388
Modular Assembly of Oligo(phenylenevinylene)s via Iterative Linear-Selective Oxidative Heck Reactions with Protected Boronic Acids
Abstract
In this paper, we report the development of an iterative cross-coupling (ICC) process utilizing oxidative Heck reactions with a protected boronic acid derivative, oxazaborolidinone, for the efficient synthesis of oligo(phenylenevinylene)s (OPVs). OPVs possess a structure characterized by alternating benzene rings and alkenes, traditionally synthesized through oligomerization of halogenated styrenes. However, this conventional approach is limited in its ability to incorporate different phenylenevinylene units into a single OPV structure. To address this challenge, we have developed a palladium-catalyzed iterative oxidative Heck reaction process employing newly synthesized oxazaborolidinone-protected boronic acids. When applied to the synthesis of structurally diverse OPVs, our methodology achieved the desired products in excellent yields, demonstrating its high efficiency and practical utility in constructing complex molecular architectures.
Figures
Similar articles
-
"We're all in it together": uniting a diverse range of professionals and people with lived experience within the development of a complex, theory-based paediatric speech and language therapy intervention.Res Involv Engagem. 2025 Jun 19;11(1):67. doi: 10.1186/s40900-025-00738-8. Res Involv Engagem. 2025. PMID: 40537841 Free PMC article.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
-
Adjustmentalisation - digitalisation as transformation process and the interplay between a digital logic and diverse primary care logics in Sweden.J Health Organ Manag. 2025 Jun 25;39(5):744-763. doi: 10.1108/JHOM-07-2024-0281. J Health Organ Manag. 2025. PMID: 39890596
-
Direct composite resin fillings versus amalgam fillings for permanent posterior teeth.Cochrane Database Syst Rev. 2021 Aug 13;8(8):CD005620. doi: 10.1002/14651858.CD005620.pub3. Cochrane Database Syst Rev. 2021. PMID: 34387873 Free PMC article.
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
References
-
-
Liquid crystals:
- Pisula W., Tomović Ž., Wegner M., Graf R., Pouderoijen M. J., Meijer E. W., Schenning A. P. H. J.. Liquid crystalline hydrogen bonded oligo(p-phenylenevinylene)s. Mater. Chem. 2008;18:2968–2977. doi: 10.1039/b803117g. - DOI
- Shimaoka E., Kunihiro M., Funahashi M.. Glass-Forming Chiral Liquid Crystalline Dimers Based on an Oligo(phenylenevinylene) Unit Exhibiting Circularly Polarized Photoluminescence. ACS Appl. Polym. Mater. 2022;4:565–574. doi: 10.1021/acsapm.1c01448. - DOI
-
Fluorescence:
- Summers M. A., Bazan G. C., Buratto S. K.. Matrix-Induced Intensity Fluctuations in the Fluorescence from Single Oligo(phenylenevinylene) Molecules. J. Am. Chem. Soc. 2005;127:16202–16206. doi: 10.1021/ja054337i. - DOI - PubMed
-
Optoelectronic devices:
- Wang S., Oldham W. J. Jr., Hudack R. A. Jr., Bazan G. C.. Synthesis, Morphology, and Optical Properties of Tetrahedral Oligo(phenylenevinylene) Materials. J. Am. Chem. Soc. 2000;122:5695–5709. doi: 10.1021/ja992924w. - DOI
-
Fluorescence:
- Nie C., Li S., Wang B., Liu L., Hu R., Chen H., Lv F., Dai Z., Wang S.. Preparation of Reactive Oligo(p-Phenylene Vinylene) Materials for Spatial Profiling of the Chemical Reactivity of Intracellular Compartments. Adv. Mater. 2016;28:3749–3754. doi: 10.1002/adma.201600106. - DOI - PubMed
-
Sensor:
- Makkad S. K.. Amine decorated polystyrene nanobeads incorporating π-conjugated OPV chromophore for picric acid sensing in water. RSC Adv. 2020;10:6497–6502. doi: 10.1039/C9RA09852F. - DOI - PMC - PubMed
-
Photovoltaic devices:
- Neuteboom E. E., Meskers S. C. J., van Hal P. A., van Duren J. K. J., Meijer E. W., Janssen R. A. J., Dupin H., Pourtois G., Cornil J., Lazzaroni R., Brédas J.-L., Beljonne D.. Alternating Oligo(p-phenylene vinylene)–Perylene Bisimide Copolymers: Synthesis, Photophysics, and Photovoltaic Properties of a New Class of Donor–Acceptor Materials. J. Am. Chem. Soc. 2003;125:8625–8638. doi: 10.1021/ja034926t. - DOI - PubMed
-
Photocatalyst:
- Dong W., Qin Z., Wang K., Xiao Y., Liu X., Ren S., Li L.. Isomeric Oligo(Phenylenevinylene)-Based Covalent Organic Frameworks with Different Orientation of Imine Bonds and Distinct Photocatalytic Activities. Angew. Chem., Int. Ed. 2023;62:e202216073. doi: 10.1002/anie.202216073. - DOI - PubMed
-
Photocatalytic hydrogen evolution:
- Dong W., Xiao Y., Qin Z., Qiao B., Li L.. Partially H-bonded covalent organic frameworks for photocatalytic hydrogen evolution. J. Mater. Chem. A. 2023;11:14760–14767. doi: 10.1039/D3TA01944F. - DOI
-
Miscellaneous:
- Moreland A. S., Limwongyut J., Holton S. J., Bazan G. C.. Structural modulation of membrane-intercalating conjugated oligoelectrolytes decouples outer membrane permeabilizing and antimicrobial activities. Chem. Commun. 2023;59:12172–12175. doi: 10.1039/D3CC02861E. - DOI - PubMed
- Gao Z., Zhang E., Zhao H., Xia S., Bai H., Huang Y., Lv F., Liu L., Wang S.. Bacteria-Mediated Intracellular Click Reaction for Drug Enrichment and Selective Apoptosis of Drug-Resistant Tumor Cells. ACS Appl. Mater. Interfaces. 2022;14:12106–12115. doi: 10.1021/acsami.2c01493. - DOI - PubMed
- Ajayaghosh A., Praveen V. K.. π-Organogels of Self-Assembled p-Phenylenevinylenes: Soft Materials with Distinct Size, Shape, and Functions. Acc. Chem. Res. 2007;40:644–656. doi: 10.1021/ar7000364. - DOI - PubMed
-
Review:
- Segura J. L., Martín N., Guldi D. M.. Materials for organic solar cells: the C60/π-conjugated oligomer approach. Chem. Soc. Rev. 2005;34:31–47. doi: 10.1039/B402417F. - DOI - PubMed
- Praveen V. K., Ranjith C., Bandini E., Ajayaghosh A., Armaroli N.. Oligo(phenylenevinylene) hybrids and self-assemblies: versatile materials for excitation energy transfer. Chem. Soc. Rev. 2014;43:4222–4242. doi: 10.1039/C3CS60406C. - DOI - PubMed
-
-
- Gillis E. P., Burke M. D.. A Simple and Modular Strategy for Small Molecule Synthesis: Iterative Suzuki–Miyaura Coupling of B-Protected Haloboronic Acid Building Blocks. J. Am. Chem. Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. - DOI - PubMed
- Lee S. J., Gray K. C., Paek J. S., Burke M. D.. Simple, Efficient, and Modular Syntheses of Polyene Natural Products via Iterative Cross-Coupling. J. Am. Chem. Soc. 2008;130:466–468. doi: 10.1021/ja078129x. - DOI - PMC - PubMed
- Gillis E. P., Burke M. D.. Multistep Synthesis of Complex Boronic Acids from Simple MIDA Boronates. J. Am. Chem. Soc. 2008;130:14084–14085. doi: 10.1021/ja8063759. - DOI - PMC - PubMed
- Knapp D. M., Gillis E. P., Burke M. D.. A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates. J. Am. Chem. Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p. - DOI - PMC - PubMed
-
Review:
- Gillis E. P., Burke M. D.. Iterative Cross-Coupling with MIDA Boronates: towards a General Strategy for Small-Molecule Synthesis. Aldrichimica Acta. 2009;42:17–27. - PMC - PubMed
-
See other challenging MIDA boronates:
- Lv W.-X., Zeng Y.-F., Li Q., Chen Y., Tan D.-H., Yang L., Wang H.. Oxidative Difunctionalization of Alkenyl MIDA Boronates: A Versatile Platform for Halogenated and Trifluoromethylated α-Boryl Ketones. Angew. Chem., Int. Ed. 2016;55:10069–10073. doi: 10.1002/anie.201604898. - DOI - PubMed
- Tan D.-T., Cai Y.-H., Zeng Y.-F., Lv W.-X., Yang L., Li Q., Wang H.. Diversity-Oriented Synthesis of α-Functionalized Acylborons and Borylated Heteroarenes by Nucleophilic Ring Opening of α-Chloroepoxyboronates. Angew. Chem. Int. Ed. 2019;58:13784–13788. doi: 10.1002/anie.201907349. - DOI - PubMed
-
-
BDAN:
- Noguchi H., Hojo K., Suginome M.. Boron-Masking Strategy for the Selective Synthesis of Oligoarenes via Iterative Suzuki–Miyaura Coupling. J. Am. Chem. Soc. 2007;129:758–759. doi: 10.1021/ja067975p. - DOI - PubMed
- Noguchi H., Shioda T., Chou C. M., Suginome M.. Differentially Protected Benzenediboronic Acids: Divalent Cross-Coupling Modules for the Efficient Synthesis of Boron-Substituted Oligoarenes. Org. Lett. 2008;10:377–380. doi: 10.1021/ol702420x. - DOI - PubMed
- Iwadate N., Suginome M.. Synthesis of masked haloareneboronic acids via iridium-catalyzed aromatic C–H borylation with 1,8-naphthalenediaminatoborane (danBH) J. Organomet. Chem. 2009;694:1713–1717. doi: 10.1016/j.jorganchem.2008.11.068. - DOI
- Iwadate N., Suginome M.. Synthesis of B-Protected β-Styrylboronic Acids via Iridium-Catalyzed Hydroboration of Alkynes with 1,8-Naphthalenediaminatoborane Leading to Iterative Synthesis of Oligo(phenylenevinylene)s. Org. Lett. 2009;11:1899–1902. doi: 10.1021/ol9003096. - DOI - PubMed
- Iwadate N., Suginome M.. Rhodium-catalyzed Dehydroborylation of Styrenes with Naphthalene-1,8-diaminatoborane [(dan)BH]: New Synthesis of Masked β-Borylstyrenes as New Phenylene–Vinylene Cross-coupling Modules. Chem. Lett. 2010;39:558–560. doi: 10.1246/cl.2010.558. - DOI
- Iwadate N., Suginome M.. Differentially Protected Diboron for Regioselective Diboration of Alkynes: Internal-Selective Cross-Coupling of 1-Alkene-1,2-diboronic Acid Derivatives. J. Am. Chem. Soc. 2010;132:2548–2549. doi: 10.1021/ja1000642. - DOI - PubMed
-
Recent progress:
- Tomota K., Li J., Tanaka H., Nakamoto M., Tsushima T., Yoshida H.. Weak Base-Promoted Direct Cross-Coupling of Naphthalene-1,8-diaminato-substituted Arylboron Compounds. J. Am. Chem. Soc. Au. 2024;4:3931–3941. doi: 10.1021/jacsau.4c00665. - DOI - PMC - PubMed
-
-
- Tsuchiya N., Nojiri T., Nishikata T.. Oxazaborolidinones: Steric Coverage Effect of Lewis Acidic Boron Center in Suzuki–Miyaura Coupling Reactions. Chem.Eur. J. 2024;30:e202303271. doi: 10.1002/chem.202303271. - DOI - PubMed
- Nojiri T., Tsuchiya N., Nishikata T.. Sterically Congested Protecting Group for a Boronyl Group in Iterative Aminations. Chem.Eur. J. 2024;30:e202303953. doi: 10.1002/chem.202303953. - DOI - PubMed
-
- Miyaura N.. Cross-Coupling Reactions: A Practical Guide. Top. Curr. Chem. 2002;219:11–59. doi: 10.1007/3-540-45313-X_2. - DOI
- Suzuki, A. ; Brown, H. C. . Organic Synthesis Via Boranes; Aldrich: Milwaukee, WI, 2003; Vol. 3.
- Miyaura N., Suzuki A.. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
LinkOut - more resources
Full Text Sources