Neoadjuvant therapy-induced immune dynamics and myeloid-associated resistance in advanced head and neck cancer
- PMID: 40483270
- PMCID: PMC12145449
- DOI: 10.1038/s41698-025-00954-1
Neoadjuvant therapy-induced immune dynamics and myeloid-associated resistance in advanced head and neck cancer
Abstract
Understanding the dynamics of the tumor-immune ecosystem is crucial for advancing neoadjuvant strategies in cancer treatment. This study investigated alterations in the tumor-immune microenvironment related to the response to preoperative combination therapy with paclitaxel, carboplatin, and cetuximab in patients with advanced head and neck squamous cell carcinoma. Thirty patients underwent combination therapy. Biopsy or surgical specimens were obtained before and after treatment. Single-cell-based, 14-marker multiplex immunohistochemistry and image cytometry were employed to assess changes in immune cell densities and profiles. Three distinct immune profiles were identified: hypo-, lymphoid-, and myeloid-inflamed. Significant decreases in tumor volume and increases in CD45+ cells and programmed cell death ligand 1 (PD-L1) scores were observed in the hypo- and lymphoid-inflamed groups, whereas the myeloid-inflamed group showed minimal changes. After treatment, increased calreticulin expression in tumor cells, together with increased lymphoid cell lineages, was observed in non-recurrent cases. The myeloid-inflamed group exhibited higher expression of hypoxia inducible factor 1α and zinc finger E-box-binding homeobox 2, suggesting the presence of a hypoxic and metastasis-promoting environment. Spatial analysis revealed that responders had a high infiltration of T cells within tumor cell nests, whereas non-responders had fewer T cells, with β-catenin expression in cancer cells. Upregulated lymphocyte activation gene 3 in the myeloid-inflammation group, and PD-L1 dynamics suggest potential targets for further therapy. These findings highlight the need for targeted neoadjuvant strategies based on immune profiling.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing non-financial interests but the following competing financial interests: T.T. is a paid consultant for Merck Biopharma and Rakuten Medical and receives speaker fees from Bristol-Myers Squibb, Merck Biopharma, Eisai Co., Ltd., Merck Sharp & Dohme Corp, Ono Pharmaceutical, Otsuka Pharmaceutical Factory, and Rakuten Medical. S.S. and H.O. are employees of SCREEN Holdings Co., Ltd. E.K. is a paid consultant for Roche Diagnostics and receives speaker fees from Chugai Pharmaceuticals. K.I. received research funding from SCREEN Holdings Co. Ltd.
Figures


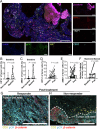
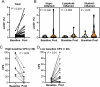
References
-
- Smith, J. D. et al. Tumor immune microenvironment alterations using induction cetuximab in a phase II trial of deintensified therapy for p16-positive oropharynx cancer. Head. Neck45, 1281–1287 (2023). - PubMed
Grants and funding
- 23K15891/Ministry of Education, Culture, Sports, Science and Technology
- 23K19516/Ministry of Education, Culture, Sports, Science and Technology
- 22K09688/Ministry of Education, Culture, Sports, Science and Technology
- JP23zf0227002/Japan Agency for Medical Research and Development
- JP23zf0227002/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

