Urine flow cytometry in older adults urinary tract infection diagnosis: is it time to reevaluate thresholds for men and women?
- PMID: 40483415
- PMCID: PMC12144684
- DOI: 10.1186/s12877-025-06063-9
Urine flow cytometry in older adults urinary tract infection diagnosis: is it time to reevaluate thresholds for men and women?
Abstract
Background: To evaluate the utility of automated urine flow cytometry in the diagnosis of urinary tract infection (UTI) in older patients without waiting for urine culture results.
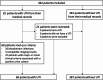
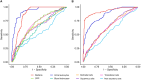
Methods: This prospective study included patients aged ≥ 65 years admitted to the emergency department of Besançon University Hospital over a six-month period. Clinical and biological data were collected and UTI diagnosis was based on strict clinical and biological criteria. Urine analysis was performed using the UF-4000 (Sysmex). Parameters or thresholds were defined based on an AUC > 0.8 and a clinically relevant negative likelihood ratio (LR-) < 0.1.
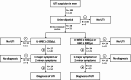
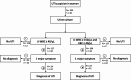
Results: Of 456 patients, 69 (15.1%) had a UTI. Bacteriuria (AUC = 0.874) and leukocyturia (AUC = 0.925) were strongly associated with UTI, with thresholds of 150 bacteria/µL and 50 leukocytes/µL (both LR- < 0.1). These cut-offs varied by sex. Urine dipsticks effectively excluded UTI in men (LR- < 0.1), but were less reliable in women (LR- = 0.129). Gender-specific diagnostic algorithms were suggested.
Conclusions: Urine flow cytometry provides valuable diagnostic thresholds for bacteriuria and leukocyturia and helps to exclude UTI before culture results. Recommendations for the diagnosis of UTI in older patients should take into account gender differences.
Keywords: Diagnosis; Elderly; Flow cytometry; Urinary tract infection; Urine culture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: The study was approved by the Institutional Review Board of Besançon University Hospital and conducted in accordance with the principles of the Declaration of Helsinki. As this was an observational study, it did not require submission to a French Institutional Ethics Committee. In accordance with the French regulatory framework (GDPR, French Data Protection Act, Public Health Code, and RGOS), patients were informed about the use of their data during hospitalization and had the right to refuse participation in any study. In France, observational studies can be conducted based on the patient’s right to object, meaning that patients must be informed about the use of their data and have the possibility to oppose it. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Wagenlehner FME, Bjerklund Johansen TE, Cai T, Koves B, Kranz J, Pilatz A, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17:586–600. - PubMed
-
- Rodhe N, Mölstad S, Englund L, Svärdsudd K. Asymptomatic bacteriuria in a population of elderly residents living in a community setting: prevalence, characteristics and associated factors. Fam Pract. 2006;23:303–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

