Clinical application of the urinary lipoarabinomannan (AIMLAM) test in PLHIV with TB
- PMID: 40483460
- PMCID: PMC12145645
- DOI: 10.1186/s12981-025-00754-4
Clinical application of the urinary lipoarabinomannan (AIMLAM) test in PLHIV with TB
Abstract
Objective: To evaluate the diagnostic performance and clinical utility of a new antigen test for the detection of urinary lipoarabinomannan (AIMLAM) in people living with HIV (PLHIV) with comorbid tuberculosis (TB).
Methods: This study included 82 PLHIV who were presumed to have TB and were admitted to Yunnan Infectious Disease Hospital from December 1, 2023, to July 1, 2024. General clinical data were collected. Urine samples were collected from all patients and subjected to AIMLAM antigen detection via chemiluminescence. Appropriate samples (such as sputum or other samples) were collected from patients in good condition for MGIT 960 mycobacterial culture (MGIT 960), GeneXpert-MTB/RIF testing (Xpert), and acid‒fast bacillus staining (AFB). According to the gold standard for outcome prediction, the patient’s history, clinical symptoms, confirmed microbiological features, and radiological/imaging findings, etc., were integrated to predict the overall outcome.
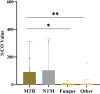
Results: Urinary AIMLAM antigen detection demonstrated greater sensitivity (83.3%) and specificity (70.7%) than MGIT 960 culture, GeneXpert-MTB/RIF, and AFB staining for diagnosing advanced HIV disease (AHD)-related TB. The diagnostic efficacy of urinary AIMLAM antigen detection was superior to that of the other tests, with an area under the curve (AUC) of 0.774, indicating significant clinical value in diagnosing TB among patients with AHD. There were 33 AIMLAM antigen-positive cases with CD4 cell counts ≤ 200 cells/µL, but the results for 14 cases were identified as false positives because of coinfections with nontuberculous mycobacteria (NTM), invasive fungal infections, or severe bacterial infections. Statistically significant differences in signal-to-cutoff ratio (S/CO) values from AIMLAM testing were observed among patients with mycobacterial, fungal, and bacterial infections (P < 0.05), but no significant difference was detected between patients with TB and those with NTM infections (P > 0.05).
Conclusion: Urine AIMLAM antigen detection is an effective tool for initial screening of TB in patients with AHD. It has higher sensitivity than MGIT 960, GeneXpert-MTB/RIF, and AFB smear, but the method needs to be optimized to increase its specificity. Positive AIMLAM results with high S/CO values suggest mycobacterial infection, although it cannot be used to differentiate TB from NTM infections. Low S/CO values in HIV patients warrant the consideration of fungal or other opportunistic infections. Species-specific confirmation is needed before treatment initiation.
Keywords: MTB; AIMLAM antigen; Lipomannan; Opportunistic infection; PLHIV; Sensitivity.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Novel automated AIMLAM for diagnosis of Mycobacterium tuberculosis.Future Microbiol. 2024 Jun 12;19(9):783-793. doi: 10.2217/fmb-2024-0025. Epub 2024 Apr 9. Future Microbiol. 2024. PMID: 38592488 Free PMC article.
-
A new strategy improving TB diagnosis: stratified urine LAM test based on lymphocyte counts.Front Cell Infect Microbiol. 2025 Feb 20;15:1498651. doi: 10.3389/fcimb.2025.1498651. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40051710 Free PMC article.
-
Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: A meta-analysis of individual in- and outpatient data.PLoS Med. 2020 May 1;17(5):e1003113. doi: 10.1371/journal.pmed.1003113. eCollection 2020 May. PLoS Med. 2020. PMID: 32357197 Free PMC article.
-
Addressing TB-related mortality in adults living with HIV: a review of the challenges and potential solutions.Ther Adv Infect Dis. 2022 Mar 18;9:20499361221084163. doi: 10.1177/20499361221084163. eCollection 2022 Jan-Dec. Ther Adv Infect Dis. 2022. PMID: 35321342 Free PMC article. Review.
-
TB screening among people living with HIV/AIDS in resource-limited settings.J Acquir Immune Defic Syndr. 2015 Apr 15;68 Suppl 3:S270-3. doi: 10.1097/QAI.0000000000000485. J Acquir Immune Defic Syndr. 2015. PMID: 25768866 Review.
References
-
- World Health Organization. Global TB report 2024 [Internet]. Geneva: World Health Organization. 2024 [cited 2025 Feb 15]. Available from: https://www.who.int/publications/i/item/global-TB-report-2024
-
- Shen YZ, Lu HZ, Chen YK, et al. Expert consensus on diagnosis and treatment of HIV/AIDS coinfected with Mycobacterium TB emerging infectious diseases electronic journal. Chin J Dermatol Venereol. 2022;7(1):73–87.
-
- World Health Organization. WHO consolidated guidelines on TB: module 3: diagnosis– [internet]apid diagnostics for TB detection [Internet]. Geneva: World Health Organization; 2020.
-
- Liu HL, Li X, Yang XP, et al. Clinical characteristics of 2992 hospitalized HIV/AIDS patients in Yunnan Province. Chin J Dermatol Venereol. 2021;35(5):525–30.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

