Plasma biomarkers for early detection of alzheimer's disease: a cross-sectional study in a Japanese cohort
- PMID: 40483474
- PMCID: PMC12144780
- DOI: 10.1186/s13195-025-01778-8
Plasma biomarkers for early detection of alzheimer's disease: a cross-sectional study in a Japanese cohort
Abstract
Background: Plasma biomarkers offer a promising alternative to amyloid beta (Aβ) positron emission tomography (PET) or cerebrospinal fluid (CSF) biomarkers for diagnosing Alzheimer's disease (AD). This cross-sectional study assessed the utility of multiple plasma biomarkers for diagnosing and staging AD in a Japanese cohort.
Methods: The assessed plasma biomarkers included Aβ42/40, phosphorylated tau (p-tau181 and p-tau217), glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL), individually and in combination. Aβ42/40 was measured using the HISCL® platform, while all other biomarkers were measured using the Simoa® platform. Participants were classified based on Aβ PET imaging and neuropsychological testing into healthy controls (HC), AD continuum (preclinical AD, mild cognitive impairment [AD-MCI], and mild dementia [AD-D]), and non-AD cognitive impairment (CI) groups. Receiver operating characteristic analyses were performed to predict the Aβ PET status, correlation with Centiloid (CL) values and cognitive scores, and biomarker comparisons across AD stages.
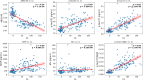
Results: Sixty-nine HC, 13 preclinical AD, 38 AD-MCI, 44 AD-D, and 79 non-AD CI participants were included. The area under the curves (AUCs) for predicting Aβ PET status were 0.937 (Aβ42/40), 0.926 (p-tau217), and 0.946 (p-tau217/Aβ42); results of pair-wise DeLong tests revealed no significant differences among these three metrics (all p > 0.05). In the cognitively normal group, the AUCs were 0.968 (Aβ42/40), 0.958 (p-tau217), and 0.979 (p-tau217/Aβ42), while in the cognitively impaired group, they were 0.919 (Aβ42/40), 0.893 (p-tau217), and 0.923 (p-tau217/Aβ42). Among HC and AD continuum participants, CL correlations were - 0.74 (Aβ42/40), 0.81 (p-tau217), and 0.83 (p-tau217/Aβ42). In the HC and AD continuum, Aβ42/40 levels showed a bimodal distribution (cutoff = 0.096), with a shift from high to low occurring at 19.3 CL, compared to the PET positivity threshold of 32.9 CL. P-tau217 exhibited a linear increase with disease progression. All biomarkers correlated strongly with logical memory scores.
Conclusions: Plasma biomarkers, Aβ42/40 and p-tau217, and particularly their ratio (p-tau217/Aβ42), show strong potential as Aβ PET alternatives for AD diagnosis. HISCL-based plasma Aβ42/40 detects Aβ accumulation earlier than Aβ PET visual reading threshold, underscoring its utility as an early diagnostic marker.
Keywords: Alzheimer’s disease; Aβ42/40; Centiloid; HISCL; Plasma biomarkers; Simoa; p-tau217; p-tau217/Aβ42.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Certified Review Board of Keio University (#N20170237) approved the study design and protocol. The study was conducted in accordance with the Declaration of Helsinki. All participants (plus their proxies as needed) provided written informed consent for participation in the study. The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; https://www.umin.ac.jp/ctr/index.htm , ID# UMIN000032027) and Japan Registry of Clinical Trials (jRCT; https://jrct.niph.go.jp/ , ID# jRCTs031180225). Consent for publication: Not applicable. Competing interests: DI has received honorariums from Daiichi Sankyo, Nihon Medi-Physics, Kowa, PDRadiopharma, Otsuka Pharmaceutical, Lilly and Eisai and has a joint research agreement with Sysmex. There are no other relationships or activities that could appear to have influenced the submitted work.
Figures





References
-
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for alzheimer’s disease. Nature. 2018;554:249–54. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
- JP17pc0101006/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

