Four months of treatment with anakinra combined with glucocorticoids for giant cell arteritis: a multicenter, randomized, double-blind, placebo-controlled trial
- PMID: 40483523
- PMCID: PMC12144695
- DOI: 10.1186/s13075-025-03493-z
Four months of treatment with anakinra combined with glucocorticoids for giant cell arteritis: a multicenter, randomized, double-blind, placebo-controlled trial
Abstract
Background: Efficacy and tolerance of anakinra (ANK) in the treatment of giant cell arteritis (GCA) need to be assessed.
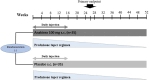
Methods: This phase 3 study (NCT02902731) was a prospective multicenter, randomized, double-blind, placebo-controlled trial conducted over a 52-week period. GCA patients were randomized 1:1. From inclusion to week 16 (W16), patients in the anakinra (ANK) group received a daily subcutaneous injection of 100 mg of anakinra, whereas patients in the other group received placebo (PBO). In both arms, glucocorticoid (GC) discontinuation was planned at week 52 (W52). The endpoints were the relapse rates at W16, W26, and W52 and the completion of GC tapering. Given the emergence of the SARS-CoV-2 pandemic, the study was stopped prematurely.
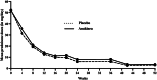
Results: Thirty patients with new GCA diagnoses from 5 centers were randomized as follows: 17 in the ANK group and 13 in the PBO group. During the first 16 weeks, the relapse rates were 12% (n = 2) and 23% (n = 3) in the ANK and PBO groups, respectively (p = 0.63). At week 26, 12 (40%) patients had relapsed: 8 (47%) in the ANK group and 4 (31%) in the PBO group (p = 0.47). At W52, the relapse rate (overall, 50%) did not differ between the ANK group (53%; 9/17 patients) and the PBO group (46%; 6/13 patients) (p = 1). Two patients in each group discontinued GCs (p = 0.87). Seven serious AEs were reported in five patients, including 4 in patients receiving ANK.
Conclusions: Although prematurely discontinued, this study does not support the use of 4 months of treatment with anakinra combined with GCs to reduce the risk of relapse or GC exposure.
Trial registration: ClinicalTrials.gov NCT02902731.
Keywords: Anakinra; Giant cell arteritis; Glucocorticoids; Interleukin-1; Quality of life.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Written informed consent was obtained from all participating patients. Consent for publication: Not applicable. Competing interests: Hubert de Boysson reports receiving fees for serving on advisory boards from Roche-Chugai and Novartis and lecture fees from Roche-Chugai, Novartis, Fresenius Kabi, GlaxoSmithKline, Amicus therapeutics, and Sanofi.
Figures
References
-
- Shick RM, Baggenstoss AH, Fuller BF, Polley HF. Effects of cortisone and ACTH on periarteritis nodosa and cranial arteritis. Proc Staff Meet Mayo Clin. 1950;25:492–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous