Modulating the activity of the APC/C regulator SISAMBA improves the sugar and antioxidant content of tomato fruits
- PMID: 40483582
- PMCID: PMC12392944
- DOI: 10.1111/pbi.70149
Modulating the activity of the APC/C regulator SISAMBA improves the sugar and antioxidant content of tomato fruits
Abstract
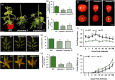
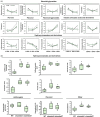
The Anaphase-Promoting Complex/Cyclosome (APC/C) is an E3 ubiquitin ligase that plays a crucial role in ubiquitin-dependent proteolysis of key cell cycle regulators, which is completed by the 26S proteasome. Previously, SAMBA, a plant-specific regulator of the APC/C, was identified in Arabidopsis as a critical factor controlling organ size through the regulation of cell proliferation. Here, by assessing its role in the crop tomato (Solanum lycopersicum), we confirm that SAMBA is a conserved APC/C regulator in plants and shows additional roles, including the modulation of fruit shape and changes in sugar metabolism. Two slsamba genome-edited lines were produced and characterized, and showed delayed growth, reduced plant size, and altered fruit morphology, which were linked to changes in cell division and expansion. Notably, untargeted metabolomics revealed altered flavonoid profiles, along with elevated Brix values in the fruits, indicating a sweeter taste. Accordingly, transcriptomics uncovered a change in temporal gene expression gradients during early fruit development, correlating with the alterations in sugar metabolism and revealing changes in cell wall biosynthesis genes. This study provides the first evidence of SAMBA's role in regulating fruit development, metabolic content, and ultimately, quality. These important findings offer potential applications for improving the nutritional quality and overall performance of tomatoes.
Keywords: APC/C; CRISPR/Ca9; fruit development; metabolic changes; tomato; transcriptomic analysis.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures






References
-
- Beauvoit, B.P. , Colombié, S. , Monier, A. , Andrieu, M.H. , Biais, B. , Bénard, C. , Chéniclet, C. et al. (2014) Model‐assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion. Plant Cell 26, 3224–3242. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2017/10333-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2020/00888-5/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2021/03212-5/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2021/06611-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2023/13107-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Medical

