In-ovo stimulation trains innate immunity to mitigate Campylobacter jejuni in broiler chickens
- PMID: 40483907
- PMCID: PMC12175704
- DOI: 10.1016/j.psj.2025.105368
In-ovo stimulation trains innate immunity to mitigate Campylobacter jejuni in broiler chickens
Abstract
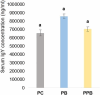
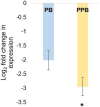
Campylobacter is the most reported zoonotic pathogen in Europe and controlling it in broiler chickens has gained much attention among the scientists especially, considering its commensalism in chicken gut. In a previous study, we demonstrated the potential of reducing Campylobacter jejuni colonization in the ceca of broiler chickens (35 days old) by in-ovo stimulation with the probiotic Leuconostoc mesenteroides B/00288 strain alone (probiotic) and in combination with garlic aqueous extract (prophybiotic). The current study further investigated the physiological and genomic responses of these birds to elucidate the potential mechanisms behind the reduction of Campylobacter observed in the ceca. The quantification of the relative abundances of selected bacterial communities in the ceca and the expression of immune-related genes in the cecal mucosa, cecal tonsils, spleen and liver was performed using qPCR. Histomorphology of the ceca was analysed by PAS staining method. The serum immunoglobulin Y (IgY) content was quantified using an ELISA method. Both treatments reduced the abundance of Akkermansia sp. and the probiotic treatment reduced Bifidobacterium sp. in the ceca of Campylobacter infected chickens when compared to the control birds (in-ovo injected with physiological saline). A higher innate immune defence response was observed through gene expression in the cecal tonsils and spleen of the in-ovo stimulated birds when compared to the control birds. The villus width and crypt depth were decreased in the in-ovo stimulated groups when compared to the control group while the serum IgY content did not differ between the groups. In conclusion, this study provides evidence that in-ovo stimulation can be an effective method in innate immunity training to control Campylobacter jejuni in broiler chickens.
Keywords: Broiler; Campylobacter jejuni; In-ovo stimulation; Innate immunity.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- AAT Bioquest I. Bioquest: quest graphTM four parameter logistic. Google Scholar. 2023 https://www.aatbio.com/tools/four-parameter-logistic-4pl-curve-regressio... Available at. (verified 9 February 2025.
-
- Alemka A., Whelan S., Gough R., Clyne M., Gallagher M.E., Carrington S.D., Bourke B. Purified chicken intestinal mucin attenuates Campylobacter jejuni pathogenicity in vitro. J. Med. Microbiol. 2010;59:898–903. - PubMed
-
- Awad W.A., Hess C.., Hess M. Re-thinking the chicken–Campylobacter jejuni interaction: a review. Avian Pathol. 2018;47:352–363. - PubMed
-
- Bhat M.I., Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017;75:374–389. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

