Differences in the autonomic regulation of temperature in the lower lip and tongue during activation of the lingual nerve
- PMID: 40483984
- PMCID: PMC12177167
- DOI: 10.1016/j.jphyss.2025.100028
Differences in the autonomic regulation of temperature in the lower lip and tongue during activation of the lingual nerve
Abstract
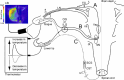
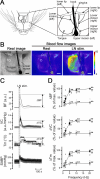
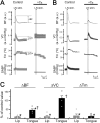
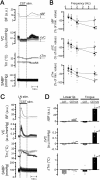
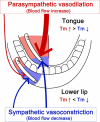
Orofacial temperature influences orofacial functions and is related to hemodynamics mediated by the autonomic nerves. Although the properties of autonomic vasomotor responses differ in orofacial tissues, differences in the autonomic regulation of orofacial temperature are unclear. We examined the differences in blood flow (BF) and temperature (Tm) between the extraoral (lower lip) and intraoral tissues (tongue) of urethane-anesthetized rats. Noncholinergic parasympathetic vasodilation evoked by trigeminal-mediated reflex elicited significant increases in BF and Tm in both tissues, and these increases were larger in the tongue than in the lower lip. Activation of cervical sympathetic nerves significantly decreased BF and Tm in both tissues. These decreases were restored by parasympathetic reflex vasodilation; the effects were larger in the tongue than in the lower lip. Our results suggest that parasympathetic vasodilation is involved in the maintenance of BF and Tm, and that the effects may be greater in intraoral than in extraoral tissues.
Keywords: Cervical sympathetic nerves; Noncholinergic parasympathetic vasodilation; Orofacial temperature; Trigeminal mediated reflex.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Effect of the parasympathetic vasodilation on temperature regulation via trigeminal afferents in the orofacial area.J Physiol Sci. 2020 Mar 31;70(1):22. doi: 10.1186/s12576-020-00749-y. J Physiol Sci. 2020. PMID: 32234014 Free PMC article.
-
Nifedipine-induced inhibition of parasympathetic-mediated vasodilation in the orofacial areas of the cat.Am J Physiol Regul Integr Comp Physiol. 2000 Jul;279(1):R332-9. doi: 10.1152/ajpregu.2000.279.1.R332. Am J Physiol Regul Integr Comp Physiol. 2000. PMID: 10896897
-
Effects of inhalation anesthetics on parasympathetic reflex vasodilation in the lower lip and palate of the cat.Am J Physiol. 1997 Jul;273(1 Pt 2):R168-74. doi: 10.1152/ajpregu.1997.273.1.R168. Am J Physiol. 1997. PMID: 9249546
-
Orthodontic treatment for crowded teeth in children.Cochrane Database Syst Rev. 2021 Dec 31;12(12):CD003453. doi: 10.1002/14651858.CD003453.pub2. Cochrane Database Syst Rev. 2021. PMID: 34970995 Free PMC article.
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

