Association of Anti-Ro52 Seropositive Interstitial Lung Disease With a Higher Risk of Disease Progression and Mortality
- PMID: 40484372
- PMCID: PMC12597573
- DOI: 10.1016/j.chest.2025.05.036
Association of Anti-Ro52 Seropositive Interstitial Lung Disease With a Higher Risk of Disease Progression and Mortality
Abstract
Background: Identifying biomarkers is vital for interstitial lung disease (ILD) management and prognostication. Although anti-Ro52 antibodies frequently are detected in autoimmune diseases, their significance in ILD remains unclear.
Research question: What is the prognostic significance of anti-Ro52 antibody positivity in patients with ILD?
Study design and methods: This retrospective cohort study used an ILD registry of patients seen at an academic tertiary hospital's ILD clinic between 2015 and 2024. All patients with a diagnosis of ILD and tested for anti-Ro52 antibody status were divided into anti-Ro52 positive and negative groups. The primary outcome was ILD progression or all-cause death. ILD progression was defined as any of the following: hospitalization because of ILD, absolute decline in FVC of ≥ 10% predicted from baseline, or lung transplantation. The Kaplan-Meier method and Cox proportional hazards regression model were used for survival analysis.
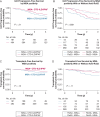
Results: Of 1,026 patients tested for the anti-Ro52 antibody (median age, 70 years; 52% male), 154 patients (15%) showed positive anti-Ro52 results. Underlying ILD subtypes were as follows: interstitial pneumonia with autoimmune features (n = 489 [48%]), connective tissue disease-associated ILD (n = 132 [13%]), idiopathic pulmonary fibrosis (n = 103 [10%]), hypersensitivity pneumonitis (n = 61 [6%]), and other idiopathic ILD (n = 241 [24%]). The anti-Ro52-positive group was younger (median age, 67 years vs 70 years), was more likely to have connective tissue disease (28% vs 10%), and more frequently showed copositive results for myositis-specific antibody (29% vs 16%). After a median follow-up of 25.6 months, patients with positive anti-Ro52 findings showed a higher risk of ILD progression or death (hazard ratio, 2.10; 95% CI, 1.61-2.73; P < .001) and showed a higher risk of lung transplantation or death (hazard ratio, 1.61; 95% CI, 1.11-2.35; P = .014) on multivariable analysis.
Interpretation: Our results indicate that Anti-Ro52-seropositive ILD is associated with significantly worse progression-free and transplant-free survival and may inform disease prognostication and monitoring.
Keywords: Anti-Ro52; ILD; TRIM21; autoantibody; interstitial lung disease.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: S. B. M. reports research funding from Pliant Therapeutics and Boehringer Ingelheim; consulting fees from Accendatech USA, DevPro Biopharma, Gilead Sciences, Mediar Therapeutics, and Roche; advisory board fees from APIE Therapeutics and Pliant Therapeutics; speaking fees from Cowen; and royalties from Wolters Kluwer. R. W. H. reports consulting fees from Vicore, Dynamed, UpToDate, Boehringer Ingelheim, Merck, and the ILD Collaborative and has served on a medical advisory board for Boehringer Ingelheim and the Myositis Association. None declared (R. I., R. S. B., S. H. Z., A. Singh, B. M. F., A. J. S., J. K. M., M. B. R., A. Soskis, B. S. S.).
Figures




References
-
- Maher T.M. Interstitial lung disease: a review. JAMA. 2024;331(19):1655–1665. - PubMed
-
- Fischer A., Antoniou K.M., Brown K.K., et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46(4):976–987. - PubMed
-
- Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. - PubMed
-
- Flaherty K.R., Wells A.U., Cottin V., et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

