Ferulic Acid Attenuates Aortic Stiffening and Cardiovascular Remodeling by Suppressing Inflammation and the Renin-Angiotensin System in Rats Fed a High-Fat/High-Carbohydrate Diet
- PMID: 40484409
- PMCID: PMC12318710
- DOI: 10.7570/jomes24017
Ferulic Acid Attenuates Aortic Stiffening and Cardiovascular Remodeling by Suppressing Inflammation and the Renin-Angiotensin System in Rats Fed a High-Fat/High-Carbohydrate Diet
Abstract
Background: Ferulic acid (FA) is an antioxidant compound present in cereals, fruits, and vegetables. Chronic consumption of a high-fat and high-carbohydrate (HFHC) diet can lead to metabolic syndrome and increase the risk of atherosclerotic cardiovascular disease. This study examined whether FA could mitigate vascular inflammation, aortic stiffness, and cardiovascular remodeling in rats fed a HFHC diet.
Methods: Male Sprague-Dawley rats were divided into five groups (eight rats/group): one group was fed a standard chow diet with or without FA supplementation, while the others were fed a HFHC diet plus a 15% fructose solution for 16 weeks. Rats on the HFHC diet received FA at doses of 0, 30, or 60 mg/kg/day during the final 6 weeks of the study. Various cardiovascular parameters, plasma biochemical markers, and the expression of biomarker proteins were measured.
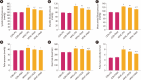
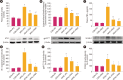
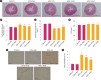
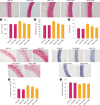
Results: FA administration alleviated the metabolic disturbances caused by the HFHC diet. FA reduced arterial blood pressure, aortic pulse wave velocity, oxidative stress, vascular inflammation, and angiotensin-mediated myocardial fibrosis and cardiac hypertrophy, as evidenced by decreases in ventricular interstitial fibrosis and cross-sectional area. These beneficial effects were associated with reduced vascular superoxide production and lower plasma levels of angiotensin-converting enzyme and tumor necrosis factor α. FA also suppressed the expression of Ang II type 1 receptor, gp91phox, and vascular-adhesion molecule 1 proteins and prevented hypertrophic remodeling of the aortic wall by reducing protein expression of matrix metalloproteinases 2 and 9.
Conclusion: This study provides insightful findings on the beneficial effects of FA in reducing aortic stiffness and cardiovascular remodeling associated with metabolic syndrome.
Keywords: Cardiovascular remodeling; Ferulic acid; Inflammation; Metabolic syndrome; Renin-angiotensin system; Vascular stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

