Nucleophilic Fluorination of a Secondary Alkyl Bromide with KF(18-Crown-6) and Bulky Diols: Microsolvation Causes Chemoselectivity Inversion in the Free Energy Profile
- PMID: 40484705
- PMCID: PMC12352739
- DOI: 10.1002/cplu.202500257
Nucleophilic Fluorination of a Secondary Alkyl Bromide with KF(18-Crown-6) and Bulky Diols: Microsolvation Causes Chemoselectivity Inversion in the Free Energy Profile
Abstract
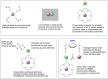
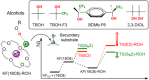
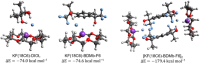
Fluoride ion solvated in polar aprotic solvents works like bases and reacts with secondary alkyl bromide substrates mainly via E2 reactions, with minor formation of SN2 fluorination products. The use of KF combined with crown ether increases the SN2 yield. However, secondary substrates remain a challenge for nucleophilic fluorination. It has recently been demonstrated that crown ethers combined with the fluorinated tert-butyl alcohol (TBOH) TBOH-F3 can increase the KF salt reactivity and selectivity toward SN2 reactions. These observations can be explained by the microsolvation of the fluoride ion with hydrogen bond donor species. This study explores computationally the effect of bulky diols in the reaction of KF(18-crown-6) with 2-bromopropane as a model substrate. This study investigates the microsolvation by TBOH, TBOH-F3, and the bulky diols pinacol and BDMb-F6, aimed at evaluating their effect on the SN2:E2 product ratio. Considering the competitive pathways, results show that TBOH is the least effective, while bulky alcohol TBOH-F3, pinacol, and BDMb-F6 favor the SN2 pathway by 0.5, 1.6, and 5.6 kcal mol-1. Thus, the study's findings indicate that pinacol and especially the BDMb-F6 diol are highly promising alcohols for achieving greater SN2:E2 selectivity in nucleophilic substitution reactions utilizing KF(18-crown-6).
Keywords: crown compounds; density functional calculations; fluorine; hydrogen bonds; solvent effects.
© 2025 The Author(s). ChemPlusChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Revealing the Mechanism of Alcohol Side Product Formation in Crown Ether-Mediated Nucleophilic Fluorination Using Acetonitrile as Solvent.ACS Omega. 2025 Jul 16;10(29):32372-32383. doi: 10.1021/acsomega.5c04699. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757364 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Effects of Hydrogen Bonding Solvation by Diverse Fluorinated Bulky Alcohols on the Reaction Rate and Selectivity in Crown Ether Mediated Nucleophilic Fluorination in an Aprotic Solvent.ACS Org Inorg Au. 2024 Nov 28;5(1):69-83. doi: 10.1021/acsorginorgau.4c00081. eCollection 2025 Feb 5. ACS Org Inorg Au. 2024. PMID: 39927103 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Gribble G. H.. Natural Production Of Organohalogen Compounds Springer‐Verlag; Berlim, Heidelberg, Germany, 2002.
-
- Verified Market Reports , “Global organofluorine compounds market size by type (fluorinated ethylene propylene (FEP) polytetrafluoroethylene (PTFE)), by application (pharmaceuticals, agrochemicals), by functionality (fluorinated intermediates, surface modifiers), by enduser industry (automotive, aerospace), by form (liquid, solid), by geographic scope and forecast.”, can be found under https://www.verifiedmarketreports.com/product/organofluorine‐compounds‐m..., (accessed: March 2025).
-
- Ragni R., Punzi A., Babudri F., Farinola G. M., Eur. J. Org. Chem. 2018, 2018, 3500.
LinkOut - more resources
Full Text Sources
Miscellaneous

