International survey of treatment practices for atopic dermatitis in pregnant and breastfeeding women: Physician perspectives
- PMID: 40484810
- PMCID: PMC12435135
- DOI: 10.1111/ddg.15728
International survey of treatment practices for atopic dermatitis in pregnant and breastfeeding women: Physician perspectives
Abstract
Background and objectives: Systemic treatment of pregnant/breastfeeding atopic dermatitis (AD) patients is challenging due to limited safety data. We explored treatment practices with systemic agents, including the guideline-recommended cyclosporine as the first systemic choice as well as emerging therapies, in this vulnerable population.
Patients and methods: The Global Allergy and Asthma Excellence Network (GA2LEN) ADCARE initiative collected data from physicians worldwide who treat pregnant women with AD. Physicians completed an electronic questionnaire on the use of systemic agents in pregnant/breastfeeding AD patients.
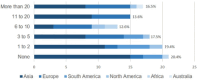
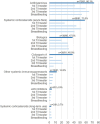
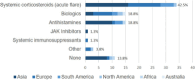
Results: 103 physicians from 32 countries completed the survey, primarily dermatologists (n = 48) or allergologists (n = 43). Antihistamines were the systemic drug most often considered to be used during pregnancy/breastfeeding (n = 73/81, 90.1%), with fewer physicians considering the use of systemic agents for the first trimester compared to later stages of pregnancy. For acute flares, systemic corticosteroids (n = 34/80, 42.5%) were preferred, followed by biologics and antihistamines (each n = 15/80, 18.8%). Although the guideline-recommended cyclosporine is sometimes considered for AD during pregnancy (n = 38/81, 46.9%), it was rarely considered as the preferred drug by physicians (n = 1/80, 1.25%).
Conclusions: Our study shows a misalignment between guideline recommendations and prescription patterns and highlights an unmet need for knowing and using the existing recommendations.
Keywords: Atopic dermatitis; breastfeeding; pregnancy; survey; systemic treatment.
© 2025 The Author(s). Journal der Deutschen Dermatologischen Gesellschaft published by John Wiley & Sons Ltd on behalf of Deutsche Dermatologische Gesellschaft.
Conflict of interest statement
M.P.P. has received research funding from Almirall; is an investigator for Allakos, Celldex Therapeutics, Incyte, Sanofi, and Trevi Therapeutics; and has received consulting fees, speaker honoraria, and/or travel fees from AbbVie, Beiersdorf, Celltrion, Eli Lilly, GA2LEN, Galderma, Menlo Therapeutics, Novartis, P.G. Unna Academy, Sanofi, StreamedUP, and Trevi Therapeutics. E.K. has acted as a speaker and advisor for Novartis, Menarini, and Pfizer. M.D.B. has been a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Amgen, Aslan, Eli Lilly, Galderma, Leo Pharma, Pfizer, Regeneron, and Sanofi‐Genzyme. N.K. has received honoraria as a speaker/consultant for Sanofi, Maruho, AbbVie, Eli Lilly Japan, Taiho Pharmaceutical, Pfizer, Mitsubishi Tanabe Pharma, Janssen Pharma, Kyowa Kirin, Celgene Japan, and Otsuka Pharmaceutical, and has received investigator‐initiated grants from Mitsubishi Tanabe Pharma, Torii Pharmaceutical, Maruho, Sun Pharma, Boehringer Ingelheim Japan, Eisai, and Leo Pharma. M.W. reports support for consultancies, lectures, and other scientific activities from ALK‐Abelló Arzneimittel GmbH, Almirall, AbbVie, Eli Lilly, Mylan Germany GmbH, Bencard Allergie GmbH, Novartis AG, Biotest AG, Sanofi‐Aventis Deutschland GmbH, HAL Allergie GmbH, DBV Technologies S.A., Aimmune Therapeutics UK Limited, Regeneron Pharmaceuticals Inc., and Stallergenes GmbH. T.W. has received institutional research grants from Almirall, Beiersdorf, LEO Pharma, and Novartis; has performed consultancies for AbbVie, Almirall, Galderma, LEO, Lilly, Novartis, Pfizer, and Sanofi‐Regeneron; has lectured at educational events sponsored by AbbVie, Almirall, Galderma, LEO Pharma, Lilly, Pfizer, Sanofi, and Novartis; and is involved in clinical trials conducted with various pharmaceutical companies developing treatments for atopic dermatitis. A.W. has served as an advisor or paid speaker for, or participated in clinical trials (with honoraria paid to the institution) sponsored by AbbVie, Aileens, Almirall, Amgen, Beiersdorf, Bioderma, Bioproject, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, DKSH, Eli Lilly, Galapagos, Galderma, Glenmark, GSK, Hans Karrer, Hexal, Janssen‐Cilag, Kyowa Kirin, Leo Pharma, L'Oréal, Maruho, MedImmune, MSD, Mylan, Novartis, Pfizer, Pierre Fabre, Regeneron, Sandoz, Santen, Sanofi‐Aventis, and UCB. T.Z. reports honoraria for lectures from Amgen, AstraZeneca, AbbVie, ALK‐Abelló, Almirall, Astellas, Bayer HealthCare, Bencard, Berlin Chemie, FAES Farma, HAL Allergie GmbH, Henkel, Kryolan, Leti, L'Oréal, Meda, Menarini, Merck Sharp & Dohme, Novartis, Nuocor, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, and Uriach. Fees for industry consulting were received from Abivax, Almirall, Bluprint, Celldex, Celltrion, Novartis, and Sanofi. In addition, he declares non‐paid organizational affiliations: committee member, “Allergic Rhinitis and its Impact on Asthma” (ARIA); member of the board, German Society for Allergy and Clinical Immunology (DGAKI); head, European Centre for Allergy Research Foundation (ECARF); president, Global Allergy and Asthma Excellence Network (GA2LEN); and member, Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO). C.Y.C. has received research funding from Sanofi; is an investigator for AbbVie, Amgen, Dermira, Janssen, Eli Lilly, Novartis, Oneness Biotech, Pfizer, Regeneron Pharmaceuticals Inc., Roche, and Sanofi; and has received consulting fees, speaker honoraria, and/or travel fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi, and Viatris. A.G.A. is or recently was a speaker and/or advisor for, and/or has received research funding from, Almirall, Amgen, AstraZeneca, Avène, Bluprint, Celldex, Escient Pharmaceuticals, Genentech, GSK, Harmonic Bio, Instituto Carlos III–FEDER, Jaspers, Leo Pharma, Menarini, Mitsubishi Tanabe Pharma, Novartis, Sanofi–Regeneron, Septerna, Servier, Thermo Fisher Scientific, Uriach Pharma, and Noucor. L.F.E. received consulting fees from Sanofi, speaker honoraria from Sanofi, Novartis, and AbbVie, and participates in clinical trials from Novartis and Amgen. K.K. received speaker honoraria from Pfizer, Zuellig Pharma, and Sanofi. E.Ö. has acted on advisory boards for Pfizer and Sanofi and has received speaker honoraria from both companies. C.R.P. is an investigator for AbbVie and has received consulting fees, speaker honoraria, and/or travel fees from AbbVie, Eli Lilly, Leo Pharma, and Janssen‐Cilag. K.S., C.M., I.V.H., P.V., L.B., D.B., H.C., K.G., M.G., S.G., C.M., N.T.M., P.P., M.M.R.F., C.A.S.P., G.R., and E.V. declare no conflicts of interest.
Figures
References
-
- Stander S. Atopic Dermatitis. N Engl J Med. 2021;384:1136‐1143. - PubMed
-
- Thyssen JP, Halling AS, Schmid‐Grendelmeier P, et al. Comorbidities of atopic dermatitis‐what does the evidence say? J Allergy Clin Immunol. 2023;151:1155‐1162. - PubMed
-
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I ‐ systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409‐1431. - PubMed

