Tumor microenvironment remodeling with a telomere-targeting agent and its cooperative antitumor effects with a nanovaccine
- PMID: 40484928
- PMCID: PMC12145632
- DOI: 10.1186/s12951-025-03471-2
Tumor microenvironment remodeling with a telomere-targeting agent and its cooperative antitumor effects with a nanovaccine
Abstract
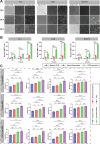
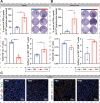
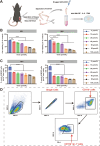
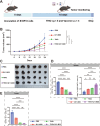
The nucleoside analogue 6-thio-2'-deoxyguanosine (6-thio-dG, also known as THIO) is a telomere-targeting agent with important clinical potency. It can selectively kill telomerase-positive tumor cells. We previously reported that THIO could successfully induce immunogenic cell death (ICD) in multiple mouse tumor cell lines. In this study, we further explored the potential impact of THIO on remodeling the tumor microenvironment, regulating anti-tumor immune responses, and its possible synergistic effects with other therapeutic methods, such as tumor vaccines. Our results showed that THIO could also induce ICD in various human tumor cell lines. The induction of ICD in tumor cells promoted the migration and maturation of antigen-presenting cells. Administration of THIO significantly inhibited the growth of established CT26 and TC-1 tumors in mice. Meanwhile, it enhanced the anti-tumor CTL response and reduced the levels of immunosuppressive myeloid-derived suppressor cells (MDSCs) in both the spleen and tumor tissues. Additionally, THIO had a direct inhibitory effect on the proliferation and differentiation of MDSCs. Moreover, when combined with bacterial biomimetic vesicles or a nanovaccine, such as THIO with BBV or different Q11-tumor antigen peptide nanofibers, it exhibited enhanced anti-tumor effects and immune responses compared to monotherapy in either "immune hot" TC-1 tumors or "immune cold" B16-F10 tumors. In summary, THIO has the ability to remodel the tumor microenvironment, exert a specific killing effect on tumor cells, and effectively cooperate with tumor vaccines. This broadens the anti-tumor mechanisms of THIO and provides a promising strategy for improving anti-tumor immunotherapies.
Keywords: 6-thio-2′-deoxyguanosine (THIO); Cancer vaccine; Immunogenic cell death (ICD); Tumor immunotherapy; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Biology, Chinese Academy of Medical Sciences. Consent for publication: All authors agreed to publish this manuscript. Competing interests: The authors declare no competing interests.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Man RJ, Chen LW, Zhu HL. Telomerase inhibitors: a patent review (2010–2015). Expert Opin Ther Pat. 2016;26(6):679–88. - PubMed
-
- Mender I, LaRanger R, Luitel K, Peyton M, Girard L, Lai TP, Batten K, Cornelius C, Dalvi MP, Ramirez M, Du W, Wu LF, Altschuler SJ, Brekken R, Martinez ED, Minna JD, Wright WE, Shay JW. Telomerase-mediated strategy for overcoming non-small cell lung cancer targeted therapy and chemotherapy resistance. Neoplasia. 2018;20(8):826–37. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82203034/National Natural Science Foundation of China
- 82073371/National Natural Science Foundation of China
- 202201AT070238/Science and Technology Project of Yunnan Province
- 2021-I2M-1-043/Chinese Academy of Medical Sciences Initiative for Innovative Medicine
- 202401BC070009/Major Project in Basic Research of Yunnan Province
LinkOut - more resources
Full Text Sources
Miscellaneous

