Co-design of a Mobile Stroke Unit pathway highlights uncertainties and trade-offs for viable system-wide implementation in the English and Welsh NHS
- PMID: 40484953
- PMCID: PMC12147245
- DOI: 10.1186/s12873-025-01243-7
Co-design of a Mobile Stroke Unit pathway highlights uncertainties and trade-offs for viable system-wide implementation in the English and Welsh NHS
Abstract
Background: Mobile stroke units (MSUs) are specialist ambulances equipped with scanning and point of care testing that can identify patients eligible for intravenous thrombolysis - medication to dissolve a clot used in ischaemic strokes - and provide this on location. While benefits of MSUs have been demonstrated, this is context dependent. Routine use of MSUs across the English and Welsh National Health Service (NHS) has not yet been considered, and as such no pathway for their operation exists. This study aimed to co-design a viable pathway, detailing dispatch, staffing and treatment decisions, for MSUs within the NHS context.
Methods: The study used interdisciplinary co-design alongside Nominal Group Technique (NGT) to generate consensus. Participants were recruited using a combination of purposive, opportunistic and snowball sampling. Data collection took place in online workshops, across three rounds, with supplemental interviews conducted where required. Data were analysed as an ongoing process, with participants checking interpretations after each round, and then further analysed deductively to identify key uncertainties following all the rounds. Consensus threshold for the NGT was set a priori at ≥ 80%.
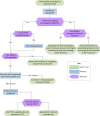
Results: An MSU pathway that reached consensus for being viable within the NHS was developed with consideration for current systems and pressures. Key uncertainties were identified such as where to base the MSU. We also identified where participants had to make trade-offs in the co-designed pathway, such as staffing considerations. Together, the uncertainties and trade-offs represent challenges to MSU implementation and are presented alongside the process to reach the finalised pathway. Future developments which may have implications for the implementation of MSUs were also explored.
Conclusions: The co-designed MSU pathway provides a foundation for MSU implementation in the English and Welsh NHS and can be subjected to local and regional modifications required for implementation. However, optimal implementation is likely hindered by several uncertainties and trade-offs, including the geographical base of the MSU and staffing, that represent challenges to implementation of MSUs at scale. Future developments in acute stroke care may help to mitigate these challenges, such as developments in artificial intelligence to read scans and improved access to telemedicine.
Clinical trial number: Not applicable.
Keywords: Co-production; Emergency medicine; Mobile stroke unit; Nominal group technique; Pathway development; Pre-hospital care; Stroke.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was provided via Northumbria University ethics online system (reference: 4117). The study was deemed by the Health Research Authority (HRA) to not require HRA approval. All participants were provided with a detailed information sheet prior to the study and provided signed consent to participate. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Stroke Association. Stroke Statistics: Stroke Association. 2021. Available from: https://www.stroke.org.uk/stroke/statistics
-
- Bluhm S, Schramm P, Spreen-Ledebur Y, Bluhm S, Münte TF, Eiersted MR, et al. Potential effects of a mobile stroke unit on time to treatment and outcome in patients treated with thrombectomy or thrombolysis: A Danish–German cross-border analysis. European Jour Neur. 2024 Sep;31(9):e16298. 10.1111/ene.16298 - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

