Targeting inflammasomes as an immunotherapeutic strategy for cancer
- PMID: 40484954
- PMCID: PMC12145604
- DOI: 10.1186/s12967-025-06665-2
Targeting inflammasomes as an immunotherapeutic strategy for cancer
Abstract
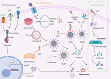
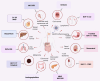
Inflammasomes are essential regulators of innate immunity, inflammation, and cellular apoptosis, and they have surfaced as significant modulators of cancer progression and regulation. Inflammasomes are macromolecular complexes assembled in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). They induce inflammation via the oligomerization and activation of caspases. These cysteine proteases cleave the pro-inflammatory cytokines IL-1β and IL-18 into their physiologically active mature versions. Recent discoveries reveal that inflammasomes are implicated not only in infections but also in malignancies, suggesting a significant connection between inflammation and tumor development. This article emphasizes that inflammasomes cause pyroptosis in a variety of immune cells, such as dendritic cells, macrophages, T cells, and fibroblasts, in addition to tumor cells. The induction of CD8+ T cells allows inflammasomes to commence an immunological response against the tumor, successfully inhibiting its growth and progression. The inflammasome comprises four main types: NLRP1, NLRP3, NLRC4, and AIM2. Nevertheless, the inflammasomes are activated by infection, injury, or stimulation of host cells, thus triggering the inflammatory response. The essential roles of the NLRP1, NLRP3, NLRC4, and AIM2 inflammasomes are emphasized in both tumors and immune cells. Furthermore, the article provides an overview of inhibitors targeting various tumor inflammasome pathways currently in clinical trials. Here, in this review, we underscore the role of the inflammatory response in cancer progression and highlight the significance of inflammasomes in regulating immune cells within the tumor microenvironment. Targeting these inflammasomes offers novel strategies for cancers.
Keywords: Immune cells; Immunotherapy; Inflammasome; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable.
Figures

Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
Role of inflammasomes in cancer immunity: mechanisms and therapeutic potential.J Exp Clin Cancer Res. 2025 Mar 29;44(1):109. doi: 10.1186/s13046-025-03366-y. J Exp Clin Cancer Res. 2025. PMID: 40155968 Free PMC article. Review.
-
Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis.Cell Mol Immunol. 2023 Dec;20(12):1513-1526. doi: 10.1038/s41423-023-01107-9. Epub 2023 Nov 27. Cell Mol Immunol. 2023. PMID: 38008850 Free PMC article.
-
Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis.Immunol Rev. 2025 Jan;329(1):e13406. doi: 10.1111/imr.13406. Epub 2024 Oct 1. Immunol Rev. 2025. PMID: 39351983 Free PMC article. Review.
-
Advances in Understanding Activation and Function of the NLRC4 Inflammasome.Int J Mol Sci. 2021 Jan 21;22(3):1048. doi: 10.3390/ijms22031048. Int J Mol Sci. 2021. PMID: 33494299 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Heras-Murillo I, Adán-Barrientos I, Galán M, Wculek SK, Sancho D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat Rev Clin Oncol. 2024;21(4):257–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

