Microbiota boost immunotherapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors
- PMID: 40484955
- PMCID: PMC12147380
- DOI: 10.1186/s12916-025-04183-y
Microbiota boost immunotherapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors
Abstract
Background: Immune checkpoint inhibitors (ICIs) are a cornerstone of modern cancer treatment, but their effectiveness is limited. Fecal microbiota transplantation (FMT), which alters the gut microbiome, has shown promise in enhancing ICIs' therapeutic effects.
Methods: We conducted a comprehensive search of relevant studies available up to September 30, 2024, to analyze the clinical efficacy and safety of combining FMT with ICIs in cancer treatment. The primary endpoint was the objective response rate (ORR), with secondary evaluations of survival outcomes and safety.
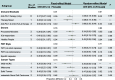
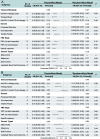
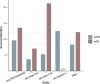
Results: A total of 10 studies involving 164 patients with solid tumors were included. The pooled ORR was 43% (95% CI: 0.35-0.51). Subgroup analysis revealed that the combination of anti-PD-1 and anti-CTLA-4 therapies was associated with a significantly higher ORR (60%) compared to anti-PD-1 monotherapy (37%; P = 0.01). The incidence of grade 1-2 adverse events (AEs) was 42% (95% CI: 0.32-0.52), while grade 3-4 AEs occurred in 37% of patients (95% CI: 0.28-0.46).
Conclusions: This meta-analysis provides preliminary evidence supporting the use of FMT as a strategy to enhance the efficacy of ICIs in patients with advanced or refractory solid tumors. However, larger-scale randomized controlled trials with long-term follow-up are required to confirm and optimize treatment protocols.
Keywords: Adverse events; Clinical efficacy; Fecal microbiota transplantation; Immune checkpoint inhibitors; Objective response rate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate.: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Fang Y, Kong Y, Rong G, Luo Q, Liao W, Zeng D. Systematic Investigation of Tumor Microenvironment and Antitumor Immunity With IOBR. Med Research. 10.1002/mdr2.70001.
Publication types
MeSH terms
Substances
Grants and funding
- 2021A1515012593/Natural Science Foundation of Guangdong Province
- 82373129/National Natural Science Foundation of China
- 82172750/National Natural Science Foundation of China
- 2022A1515111212/Basic and Applied Basic Research Foundation of Guangdong Province
- 2023A04J1257/Science and Technology Program of Guangzhou
LinkOut - more resources
Full Text Sources
Medical

