Perfusate Biomarker Comparison During Renal Hypothermic and Normothermic Machine Perfusion: Do These Techniques Provide Similar Insights?
- PMID: 40485005
- PMCID: PMC12453108
- DOI: 10.1097/TP.0000000000005440
Perfusate Biomarker Comparison During Renal Hypothermic and Normothermic Machine Perfusion: Do These Techniques Provide Similar Insights?
Abstract
Background: Hypothermic machine perfusion (HMP) and normothermic machine perfusion (NMP) are increasingly used in renal transplantation. Both techniques enable pretransplant organ viability assessment through biomarker measurements in the perfusion solution. This study examines similarities and differences in biomarker release during HMP and NMP, focusing on well-established biomarkers alongside functional markers in porcine and discarded human donor kidneys.
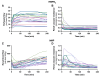
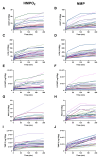
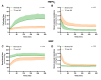
Methods: Discarded human donor kidneys (n = 25) underwent 4 h of oxygenated hypothermic machine perfusion (HMPO 2 ) and subsequently 4 h of NMP. Porcine kidneys were exposed to either minimal warm ischemia or 75 min of warm ischemia (n = 30 per group). Hereafter, kidneys were placed on HMPO 2 for 6 h followed by 6 h of NMP. Flow dynamics were recorded, and the biomarkers aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), N -acetyl-β-glucosaminidase, tissue inhibitor of metalloproteinases-2 (TIMP-2), and heart-type fatty acid-binding protein were measured longitudinally in the perfusates.
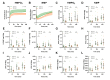
Results: For human kidneys, we found moderate to strong correlations between ASAT, LDH, TIMP-2, and heart-type fatty acid-binding protein content measured during HMPO 2 and the same biomarkers during NMP. In porcine kidneys, clear distinctions between ischemically damaged and healthy kidneys were observed in flow dynamics and content of ASAT, LDH, and TIMP-2 during both HMPO 2 and NMP.
Conclusions: Our findings suggest that biomarker release during HMPO 2 and NMP have similarities, indicating that some biomarkers might already be assessed during HMPO 2 . However, the predictive value of biomarkers in both techniques remains elusive. Additionally, NMP could provide important benefits over HMPO 2 , including functional assessment and reconditioning.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
C.L.J. is co-founder and CEO of 34Lives. H.G.D.L is part-time CSO of 34Lives. The other authors declare no conflicts of interest.
Figures




References
-
- Moers C, Smits JM, Maathuis MHJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. - PubMed
-
- Moers C, Pirenne J, Paul A, et al. ; Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2012;366:770–771. - PubMed
-
- Jochmans I, Moers C, Smits JM, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. 2011;11:2214–2220. - PubMed
-
- Moers C, Varnav OC, Van Heurn E, et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation. 2010;90:966–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

