Treatment of Hypovitaminosis D With Cholecalciferol in Dogs With Protein-Losing Enteropathies: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial
- PMID: 40485009
- PMCID: PMC12146210
- DOI: 10.1111/jvim.70147
Treatment of Hypovitaminosis D With Cholecalciferol in Dogs With Protein-Losing Enteropathies: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial
Erratum in
-
Erratum to "Treatment of Hypovitaminosis D With Cholecalciferol in Dogs With Protein-Losing Enteropathies: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial".J Vet Intern Med. 2025 Sep-Oct;39(5):e70212. doi: 10.1111/jvim.70212. J Vet Intern Med. 2025. PMID: 40844077 Free PMC article. No abstract available.
Abstract
Background: The effects of vitamin D supplementation are unknown in dogs with protein-losing enteropathy (PLE).
Objective: To evaluate the safety, efficacy, and clinical benefit of orally administered cholecalciferol in dogs with PLE and decreased serum concentrations of 25OHD.
Animals: Twenty-eight dogs with PLE, decreased 25OHD, and serum ionized calcium (iCa) > 1.0 mmol/L (n = 15 treated with cholecalciferol, n = 13 treated with placebo).
Methods: Prospective, double-blinded, randomized, controlled trial. Dogs randomized to receive 400 IU/kg cholecalciferol or placebo PO daily along with standard therapy for 6 weeks. Clinical and biochemical variables were measured at baseline (T0) and monitored at 2 (T1), 4 (T2), and 6 (T3) weeks postmedication initiation. Clinical and biochemical variables were also measured 6 weeks following discontinuation of study medication (T4). Variables were compared in dogs with PLE receiving cholecalciferol versus placebo at T0-T4 using Student's t test or Mann-Whitney U tests and a mixed-effects model. Correlations between 25OHD and clinical and biochemical variables were also performed.
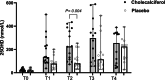
Results: Dogs with PLE treated with cholecalciferol had higher 25OHD concentrations at T2 compared to dogs treated with placebo (225 nmol/L, range 72-434 vs. 80 nmol/L, range 31-254 nmol/L; p = 0.004). Clinical and biochemical variables did not otherwise differ between dogs with PLE treated with cholecalciferol versus placebo at T0-T4. Serum albumin correlated with 25OHD at T0-T3(p < 0.005 for all comparisons). Hypervitaminosis D without ionized hypercalcemia occurred in five dogs (18%).
Conclusions: While PLE dogs treated with cholecalciferol had higher 25OHD concentrations at study timepoints, a clinical benefit of supplementation was not observed.
Keywords: 25OHD; canine; cholecalciferol; protein‐losing enteropathy; vitamin D.
© 2025 The Author(s). Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

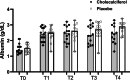
References
-
- Dossin O. and Lavoué R., “Protein‐Losing Enteropathies in Dogs,” Veterinary Clinics of North America. Small Animal Practice 41, no. 2 (2011): 399–418. - PubMed
-
- Allenspach K. and Iennarella‐Servantez C., “Canine Protein Losing Enteropathies and Systemic Complications,” Veterinary Clinics of North America. Small Animal Practice 51, no. 1 (2021): 111–122. - PubMed
-
- Gow A. G., Else R., Evans H., Berry J. L., Herrtage M. E., and Mellanby R. J., “Hypovitaminosis D in Dogs With Inflammatory Bowel Disease and Hypoalbuminaemia,” Journal of Small Animal Practice 52, no. 8 (2011): 411–418. - PubMed
-
- Mellanby R. J., Mellor P. J., Roulois A., et al., “Hypocalcaemia Associated With Low Serum Vitamin D Metabolite Concentrations in Two Dogs With Protein‐Losing Enteropathies,” Journal of Small Animal Practice 46, no. 7 (2005): 345–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

