Effect of glucagon-like peptide-1 receptor agonist on paclitaxel induced neurotoxicity in dorsal root ganglion neuronal cells in vitro
- PMID: 40485178
- PMCID: PMC12221959
- DOI: 10.3344/kjp.24419
Effect of glucagon-like peptide-1 receptor agonist on paclitaxel induced neurotoxicity in dorsal root ganglion neuronal cells in vitro
Abstract
Background: Chemotherapy is the basis of cancer treatment. Chemotherapy-induced peripheral neuropathy (CIPN) is a major adverse effect. CIPN has a high incidence rate and substantially affects the quality of life of cancer survivors. Despite this, no definitive treatment for persistent unmet medical needs is available. Glucagon-like peptide-1 receptor agonists (GLP-1RA), widely used to treat obesity and diabetes, have recently been reported to be efficacious in treating neuropathic pain. The authors aimed to confirm its effects on CIPN and elucidate its underlying mechanisms.
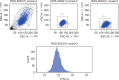
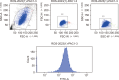
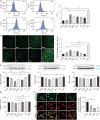
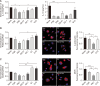
Methods: After differentiation, 50B11 dorsal root ganglion cells were treated with paclitaxel and GLP-1RA to confirm changes in oxidative stress, neuroinflammation, and neuronal damage. Immunofluorescence, flow cytometry, Western blotting, quantitative reverse transcription-polymerase chain reaction, cytokine quantitation by ELISA, and assessments of axonal degeneration and regeneration were performed to evaluate the effect of GLP-1RA on CIPN and confirm the associated mechanisms.
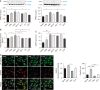
Results: After treatment with paclitaxel, the increased oxidative stress and inflammatory signals decreased with the administration of GLP-1RA. GLP-1RA also led to an increase in β-endorphin and μ-opioid receptors through IL-10. And administration of GLP-1RA had the effect of increasing neurite length. Additionally, the increased expression of the TRP family induced by paclitaxel treatment was restored with GLP-1RA administration.
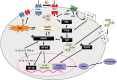
Conclusions: GLP-1RA reduces oxidative stress and neuroinflammation, thereby alleviating paclitaxel-induced neurotoxicity. Additionally, GLP-1RA increases β-endorphin and μ-opioid receptors and reduces TRP family expression and promotes neuroregeneration, suggesting its effectiveness in mitigating chemotherapy-induced neuropathic pain.
Keywords: Drug Therapy; Exenatide; Ganglia; Glucagon-Like Peptide 1; Nerve Regeneration; Neuroinflammatory Diseases; Oxidative Stress; Paclitaxel; Spinal; beta-Endorphin.
Conflict of interest statement
Eunsoo Kim is a section editor of the Korean Journal of Pain. However, he was not involved in the selection of peer reviewers, the evaluation, or the decision-making process for this article.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

