Metal-Mediated Il-8 Binding to Heparan Sulfate Evaluated by Electrochemical Impedance Spectroscopy
- PMID: 40485192
- PMCID: PMC12238926
- DOI: 10.1002/chem.202501011
Metal-Mediated Il-8 Binding to Heparan Sulfate Evaluated by Electrochemical Impedance Spectroscopy
Abstract
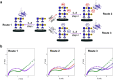
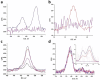
Heparan sulfate (HS) interactions with interleukin 8 (IL-8) are crucial for immune system response. The structural features of the HS and the environmental entities, such as metal ions, can regulate these interactions. However, it is challenging to evaluate the effect of each parameter on the interactions because of low accessibility to well-defined saccharides and the lack of characteristic features to be determined by analytical tools. We evaluated the effect of the HS structural features on IL-8 binding affinity utilizing electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS). We showed that the metal ions Ca2+ and Mg2+ dissimilarly mediate the interactions of HS and IL-8 in structure-dependent manner of the HS. We showed that in all glycans, a positive synergistic effect on IL-8 binding was observed. For several glycans, the presence of ions resulted in a dramatic increase in the affinity to IL-8, while for other glycans, a milder effect was observed. This demonstrated that both structural motifs and environmental features are crucial for maintaining the interactions between the HS and IL-8.
Keywords: EIS; X‐ray photoelectron spectroscopy; heparan sulfate; interleukin 8; metal ions.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
-
- Xie M., ping Li J., Cell. Signal. 2019, 54, 115. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

