Intracellular trafficking of furin enhances cellular intoxication by recombinant immunotoxins based on Pseudomonas exotoxin A
- PMID: 40485510
- PMCID: PMC12208400
- DOI: 10.1242/bio.061792
Intracellular trafficking of furin enhances cellular intoxication by recombinant immunotoxins based on Pseudomonas exotoxin A
Abstract
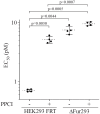
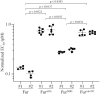
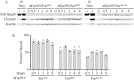
Furin is a mammalian serine protease with important roles in cellular homeostasis and disease. It cleaves and activates numerous endogenous and exogenous substrates, including the SARS-CoV-2 viral spike protein and protein toxins such as diphtheria toxin and Pseudomonas exotoxin A (PE). Recombinant immunotoxins (RITs) are toxin conjugates used as cancer therapeutics that connect tumor-directed antibodies with toxins for targeted cell killing. RITs based on PE have shown success in treating a variety of cancers, but often suffer from safety and efficacy concerns when used clinically. We have explored furin as a potential limiting factor in the intoxication pathway of PE-based RITs. Although the furin has widely recognized importance in RIT intoxication, its role is incompletely understood. Circumstantial evidence suggests that furin may act as a transporter for RITs in addition to its role of activation by cleavage. Here, we describe the creation of a CRISPR-engineered furin-deficient HEK293 cell line, ΔFur293. Using ΔFur293 and derivatives that express mutant forms of furin, we confirm the importance of furin in the PE RIT intoxication pathway and show that furin trafficking has a significant impact on RIT efficacy. Our data support the hypothesis that furin acts as a transporter during RIT intoxication and suggest furin as a target for modification to improve the effectiveness of RITs.
Keywords: Pseudomonas exotoxin A; Cancer therapy; Furin; Gene knockout; Intracellular trafficking; Recombinant immunotoxin.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Designing the furin-cleavable linker in recombinant immunotoxins based on Pseudomonas exotoxin A.Bioconjug Chem. 2015 Jun 17;26(6):1120-8. doi: 10.1021/acs.bioconjchem.5b00190. Epub 2015 Jun 3. Bioconjug Chem. 2015. PMID: 25997032 Free PMC article.
-
Novel EGFR-specific immunotoxins based on panitumumab and cetuximab show in vitro and ex vivo activity against different tumor entities.J Cancer Res Clin Oncol. 2015 Dec;141(12):2079-95. doi: 10.1007/s00432-015-1975-5. Epub 2015 Apr 22. J Cancer Res Clin Oncol. 2015. PMID: 25899161 Free PMC article.
-
In vitro anti-tumor activities of a novel recombinant immunotoxin targeting differentially overexpressed Leucine-rich repeat-containing G-protein-coupled receptor 5 in cervical cancer.Immunopharmacol Immunotoxicol. 2025 Aug;47(4):450-459. doi: 10.1080/08923973.2025.2504904. Epub 2025 May 26. Immunopharmacol Immunotoxicol. 2025. PMID: 40376858
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Batra, J. K., Fitzgerald, D. J., Chaudhary, V. K. and Pastan, I. (1991). Single-chain immunotoxins directed at the human transferrin receptor containing Pseudomonas exotoxin A or diphtheria toxin: anti-TFR(Fv)-PE40 and DT388-anti-TFR(Fv). Mol. Cell. Biol. 11, 2200-2205. 10.1128/mcb.11.4.2200-2205.1991 - DOI - PMC - PubMed
-
- Becker, G. L., Lu, Y., Hardes, K., Strehlow, B., Levesque, C., Lindberg, I., Sandvig, K., Bakowsky, U., Day, R., Garten, W., et al. (2012). Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases. J. Biol. Chem. 287, 21992-22003. 10.1074/jbc.M111.332643 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

