Impact of human papillomavirus vaccines in the reduction of infection, precursor lesions, and cervical cancer: A systematic literature review
- PMID: 40485552
- PMCID: PMC12153211
- DOI: 10.1080/21645515.2025.2497608
Impact of human papillomavirus vaccines in the reduction of infection, precursor lesions, and cervical cancer: A systematic literature review
Abstract
Cervical cancer is a preventable disease for which vaccines are available to provide long-term protection against human papillomavirus (HPV) infection. This systematic literature review (SLR) was performed to summarize the efficacy, effectiveness, impact, duration of protection, and safety profile of four licensed HPV vaccines against infection, precursor lesions, and cervical cancer. Data was extracted from published reports. The search resulted in 1,136 studies, of which 54 were selected for this review. A substantial decrease in the prevalence of oncogenic HPV types, high-grade cervical lesions, and cervical cancer was found in countries with high vaccine coverage and routine vaccination programs. Post-licensure studies of HPV vaccines have reported high efficacy, effectiveness, and health impact across settings and age groups. Studies emphasize vaccination in younger age groups. These findings may inform future discussions about HPV vaccination strategies.
Keywords: HPV vaccine; Human papillomavirus; cervical cancer; cervical intraepithelial neoplasia; systematic literature review.
Conflict of interest statement
Xavier Bosch declares receiving consulting fees and payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events, as well as support for attending meetings and/or travel once a year from MSD. Xavier Bosch also declares receipt of HPV testing kits and reagents from RMS. Peter Sasieni declares receiving grants from Cancer Research UK, NIHR and Yorkshire Cancer Research as well as a contract with GRAIL. He also declares receiving consulting fees from GRAIL for being a Scientific Advisory Board member and from Roche for a Scientific Advisory role. Peter Sasieni also received a supply of Gardasil-9 for the NOVEL trial on which he is the lead statistician from MSD. Margaret Stanley declares having acted as a consultant for GSK and MSD vaccines. Yara Ruiz was a GSK employee when the study was conducted and holds financial equities in GSK. Laura Vallejo-Aparicio and Laura Martín-Gomez are GSK employees and hold financial equities in GSK. Andrea García is a GSK employee. Angela Rodriguez and Helena Carrión were GSK employees when the study was conducted. María Yébenes and Néstor Martínez are Pharmacoeconomics & Outcomes Research Iberia (PORIB) employees. PORIB is a consultant company specialized in economic evaluation of health technologies which has received unrestricted financial support for development of the present study. Diane Harper declares receiving fees for being a consultant on health economics for Roche and for being the president of NAPCRG and funding from the National Cancer Institute (P30CA046592-29-S4 and the National Center for Advancing Translational Sciences: UL1TR001070). José Antonio Navarro-Alonso and Jorma Paavonen declare no financial and non-financial relationships and activities and no conflicts of interest. Andrea García, Angela Rodriguez, Diane Harper, Helena Carrión, Laura Vallejo-Aparicio, Laura Martín-Gomez, Margaret Stanley, María Yébenes, Néstor Martínez, Peter Sasieni, Xavier Bosch and Yara Ruiz declare no other financial and non-financial relationships and activities.
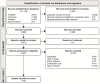
Figures
References
-
- World Health Organization Cervical Cancer . 2024. [accessed 2022 Nov 11]. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical