Early Determination of Tacrolimus Concentration-Dose Ratio Identifies Risk of Allograft Loss in Kidney Transplantation
- PMID: 40485683
- PMCID: PMC12142798
- DOI: 10.1016/j.ekir.2025.02.014
Early Determination of Tacrolimus Concentration-Dose Ratio Identifies Risk of Allograft Loss in Kidney Transplantation
Abstract
Introduction: Fast tacrolimus-metabolizing kidney transplant recipients (KTRs) (i.e., tacrolimus trough-level/total daily dose [C0/D < 1.05]) have poorer allograft function; however, their identification in a real-life setting is challenging. We investigated the reproducibility of tacrolimus metabolic status during the first months after transplantation and its association with long-term allograft outcomes.
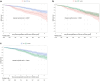
Methods: All KTRs between 2000 and 2019 with a functional allograft at 1 month and receiving tacrolimus in our center were included. Fast or slow tacrolimus metabolizers were classified according to the time spent with a C0/D < 1.05 (> 75% = High, < 25% = Low) at various time points posttransplantation. We first determined the earliest accurate time for patient categorization by investigating C0/D variability during the first months. Second, a multivariate cause-specific Cox model studying allograft outcomes was performed in groups identified by their status determined from the earliest accurate timepoint after transplantation.
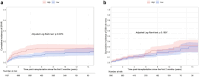
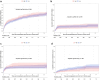
Results: Among 1979 patients included in the analysis, 2 months was the earliest accurate timepoint to determine High patients (85% of High patients identified at 2 months remained High long-term, Brier score = 0.06). Multivariate analysis revealed that High patients determined at 2 months (n = 499) had a significantly higher risk of allograft loss (cause-specific hazard ratio [CS-HR] = 2.00, 95% confidence interval [CI] = 1.48-2.69) and allograft rejection (CS-HR = 1.71, 95% CI = 1.15-2.54) than Low patients after adjustment for confounding factors. Moreover, allograft function was lower in High patients (46.7 vs. 52.9 ml/min, at 3 years, P < 0.0001) with a higher proportion of chronic vascular lesions at 1 year.
Conclusion: C0/D is a simple and pragmatic tool capable of identifying patients at risk of rejection and allograft failure as early as the second month posttransplantation.
Keywords: allograft survival; kidney transplantation; tacrolimus toxicity.
© 2025 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
-
- Gatault P., Kamar N., Büchler M., et al. Reduction of extended-release tacrolimus dose in low-immunological-risk kidney transplant recipients increases risk of rejection and appearance of donor-specific antibodies: A randomized study. Am J Transplant. 2017;17:1370–1379. doi: 10.1111/ajt.14109. - DOI - PubMed
LinkOut - more resources
Full Text Sources

