GPX4 activator enhances neuroprotection and functional recovery in spinal cord injury
- PMID: 40485847
- PMCID: PMC12143174
- DOI: 10.1016/j.jot.2025.03.013
GPX4 activator enhances neuroprotection and functional recovery in spinal cord injury
Abstract
Background: Spinal cord injury (SCI) exerts severe physical, social, and economic effects on individuals and the healthcare system. While much progress has been made in understanding the pathophysiology of SCI, the regulation of the ferroptosis master regulator, GPX4 (Glutathione Peroxidase 4), remains poorly understood.
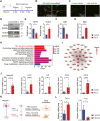
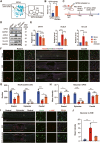
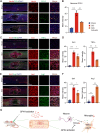
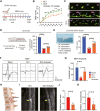
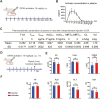
Methods: In a rat T10 contusion SCI model, GPX4 expression was tracked with western blot and immunofluorescence. Ferroptosis was induced in primary neurons using the GPX4 inhibitor RSL3, and inflammatory cytokine release was measured. Conditioned media from these neurons was applied to microglia to assess activation. The GPX4 activator PKUMDL-LC-102 was administered to SCI rats, with functional recovery evaluated through behavioral tests, MRI, and motor-evoked potentials.
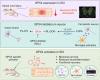
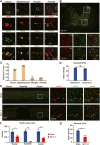
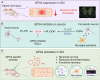
Results: We first reveal a temporal and spatial decrease of GPX4 levels in neurons after SCI. We then demonstrate that GPX4 inhibition leads to primary neuronal ferroptosis, triggering the secretion of pro-inflammatory cytokines that activate microglia. This study represents the initial in vivo investigation of GPX4-specific targeted activation, demonstrating its potential to promote functional recovery in contusive SCI by improving neuronal survival and reducing microgliosis.
Conclusion: These findings highlight the significance of GPX4 as a key factor for neuroprotection in the spinal cord. We identified the pivotal role of GPX4 in SCI and realize the neuroprotection via specific GPX4 activation to improve functional recovery in vivo.
The translational potential of this article: These findings provide a novel avenue for therapeutic intervention to enhance functional recovery after SCI through GPX4 targeted activation.
Keywords: Ferroptosis; GPX4 activation; Neuroinflammation; Neuroprotection; Spinal cord injury.
© 2025 The Authors.
Conflict of interest statement
X.L, Y.P, and B.F. contributed equally to this work. X.L.,Y.P., and B.F. co-designed the study, conducted experiments, analyzed and interpreted the data, co-made the figures, and co-wrote the manuscript. Y.P., J.Z., and X.L. conducted SCI surgery and western blotting. B.F. assisted in neuron cultures and provided RNA sequencing data. C.Z. and T.Z. performed analyses. Y.L. assisted with small molecule biosafety study. X.Z. performed LC-MS/MS analysis of redox metabolites. Y.L. and L.L. assisted in GPX4 activator studies. X.W. and G.N. co-supervised the project and contributed to experimental design, interpretation, and manuscript editing. X.Y., W.L., and S.F. supervised the project, guided experimental design and interpretation, co-made the figures, and co-wrote the manuscript. All authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This declaration serves to demonstrate our commitment to scientific ethics and to ensure the trustworthiness of our research findings.
Figures

References
-
- Squair J.W., Gautier M., Mahe L., Soriano J.E., Rowald A., Bichat A., et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature. 2021;590(7845):308–314. - PubMed
-
- Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

