Assessment of feasibility of actigraphy as a measure of clinical change in response to an experimental interventional treatment in adolescents and adults with autism spectrum disorder
- PMID: 40485939
- PMCID: PMC12143325
- DOI: 10.3389/fpsyt.2025.1570611
Assessment of feasibility of actigraphy as a measure of clinical change in response to an experimental interventional treatment in adolescents and adults with autism spectrum disorder
Abstract
Objective: The use of actigraphy as a continuous experimental measure of clinical change was explored through a comparison of two clinical studies in autism spectrum disorder (ASD). The data quality, implementation ease, wear compliance, and clinical outcome correlation of actigraphy as a measure were assessed.
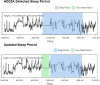
Methods: Two clinical studies were conducted and used as a basis of comparison: (1) AUT2001, a Phase 2A interventional study in ASD (N=63), and (2) AUT0002, a Phase 0 non-interventional study in typically-developing (TD) participants (N=53). Participants in both studies wore a wrist-based actigraph throughout enrollment. Actigraphy features were identified based on potential clinical relevance and calculated as weekly averages for each participant's study timepoints. Expert review was used to confirm validity of automated sleep/wake period detection. Feature differences were then assessed using t tests/ANCOVA. Spearman rank correlations between actigraphy features and caregiver reported outcome measures were also examined.
Results: Results from both clinical studies were combined during analysis. Actigraphy was shown to be feasible as a measure of longitudinal change in ASD, but with notable challenges in adherence: participant exclusions due to poor wear compliance substantially reduced the size of the final dataset. Despite this limitation, several findings were noted. Significant differences in sleep disturbance were observed at baseline between the ASD and TD populations as measured by physical activity occurring within the defined sleep period. No significant between-group differences were noted in changes from baseline to endpoint in key sleep variables. Caregiver reported sleep quality significantly correlated with actigraphy measures of sleep disturbance. Additional significant correlations were observed between caregiver reported outcomes of self-regulation and actigraphy features measuring daytime physical activity. Finally, potentially relevant correlations with anxiety, social responsiveness, and restricted and repetitive behaviors are reported.
Conclusions: The observed correlations suggest there may be alignment between some generalized features of actigraphy and core and associated domains of ASD. The clinical utility of actigraphy as a biomarker of clinically relevant outcomes in ASD requires further study. Actigraphy may provide supportive evidence of treatment outcome, providing clinical context, or as a objective behavioral measure (e.g., of sleep or activity level) when combined with more traditional clinical outcome measures.
Keywords: actigraphy; autism spectrum disorder; biomarker; clinical trials; feasibility; outcome measurement.
Copyright © 2025 Boice, Bryant, Klein, Meyer, Bangerter, Vairavan and Pandina.
Conflict of interest statement
MB, MM, AB, SV, and GP are employed by Janssen Research & Development, LLC and may hold company stock/stock options. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Relationship Between Sleep and Behavior in Autism Spectrum Disorder: Exploring the Impact of Sleep Variability.Front Neurosci. 2020 Mar 24;14:211. doi: 10.3389/fnins.2020.00211. eCollection 2020. Front Neurosci. 2020. PMID: 32265629 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Age-Related Differences in Accelerometer-Assessed Physical Activity and Sleep Parameters Among Children and Adolescents With and Without Autism Spectrum Disorder: A Meta-Analysis.JAMA Netw Open. 2023 Oct 2;6(10):e2336129. doi: 10.1001/jamanetworkopen.2023.36129. JAMA Netw Open. 2023. PMID: 37801316 Free PMC article. Review.
-
Feasibility of Actigraphy for Evaluating Sleep and Daytime Physical Activity in Children with Autism Spectrum Disorder.J Autism Dev Disord. 2023 Sep;53(9):3670-3682. doi: 10.1007/s10803-022-05661-5. Epub 2022 Jul 13. J Autism Dev Disord. 2023. PMID: 35829946
-
Application of a novel actigraphy algorithm to detect movement and sleep/wake patterns in children with autism spectrum disorder.Sleep Med. 2020 Jul;71:28-34. doi: 10.1016/j.sleep.2020.02.020. Epub 2020 Mar 6. Sleep Med. 2020. PMID: 32454300 Free PMC article.
References
-
- US Food & Drug Administration . Framework for the use of digital health technologies in drug and biological product development (2023). Available online at: https://www.fda.gov/media/166396/download (Accessed April 1, 2025).
-
- Lehrer HM, Yao Z, Krafty RT, Evans MA, Buysse DJ, Kravitz HM, et al. . Comparing polysomnography, actigraphy, and sleep diary in the home environment: The Study of Women’s Health Across the Nation (SWAN) Sleep Study. SLEEP Adv. (2022) 3:zpac001. doi: 10.1093/sleepadvances/zpac001 - DOI - PMC - PubMed
-
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. . Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. (2007) 30(4):519–29. doi: 10.1093/sleep/30.4.519. - DOI - PubMed
-
- Peis I, López-Moríñigo JD, Pérez-Rodríguez MM, Ruiz-Gómez M, Artés-Rodríguez A, Baca-García A, et al. . Actigraphic recording of motor activity in depressed inpatients: a novel computational approach to prediction of clinical course and hospital discharge. Sci Rep. (2020) 10:17286. doi: 10.1038/s41598-020-74425-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

