The approach to placement of a continuous-flow intracorporeal ventricular assist device in small left ventricle
- PMID: 40486118
- PMCID: PMC12142627
- DOI: 10.1016/j.jhlto.2025.100256
The approach to placement of a continuous-flow intracorporeal ventricular assist device in small left ventricle
Abstract
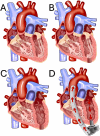
Background: The HeartMate 3 (HM3) has become among the most widely utilized durable left ventricular (LV) assist devices (LVAD) owing to reduced rates of pump thrombosis, bleeding, and stroke. One limitation of the HM3 is its large size, which poses a challenge for implantation in smaller LVs. Herein, we describe a novel technique for durable LVAD implantation in patients with a small LV.
Methods: Patients who underwent durable LVAD implantation from January 2020 to August 2024 were included in this study. The modified technique involved excision of the mitral valve (MV) and associated apparatus to create room for the LVAD inflow. The primary outcome was mortality, and secondary outcomes included rates of postoperative complications and hemodynamic parameters. Patient follow-up was until September 2024.
Results: Eleven patients were included in this study. All patients received an HM3. The median preoperative LV end-diastolic diameter was 5.1 cm. The median total time on LVAD was 149 days, and overall mortality was 27.2% occurring a median of 204 days post-LVAD implantation. Four patients (36.4%) underwent heart transplantation and 4 (36.4%) were alive on LVAD at last follow-up. Proportions of morbidity included readmission for heart failure (n = 2, 18.2%), cerebrovascular accident (n = 2, 18.2%), and pump thrombosis (n = 0).
Conclusions: The small LV has been a significant challenge for durable LVAD insertion and is often considered a contraindication. A modified approach to LVAD insertion, including excision of the MV and associated apparatus and alignment of the LVAD inflow cannula with the MV orifice, allows for LVAD implantation in patients with a small LV.
Keywords: heart failure; left ventricle; mechanical circulatory support; mitral valve; ventricular assist device.
© 2025 International Society for Heart and Lung Transplantation.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Holger Buchholz reports a relationship with Abbott that includes consulting or advisory. Jennifer Conway reports a relationship with Abbott that includes funding grants. Jennifer Conway: medical monitor of the Pumpkin Trial. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgments and Funding: None.
Figures


References
-
- Mehra M.R., Uriel N., Naka Y., et al. A fully magnetically levitated left ventricular assist device—final report. New Eng J Med. 2019;380(17):1618–1627. - PubMed
-
- Schmitto J.D., Mariani S., Li T., et al. Five-year outcomes of patients supported with HeartMate 3: a single-centre experience. Eur J Cardio-Thorac Surg. 2021;59:1155–1163. - PubMed
-
- Saeed D., Feldman D., Banayosy A.El, et al. The 2023 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: a 10-year update. J Heart Lung Transplant. 2023;42:e1–e222. - PubMed
-
- Stawiarski K., Stulak J.M., Ramakrishna H. HeartMate 3—analysis of recent trial data. J Cardiothorac Vasc Anesth. 2021;35:3105–3107. - PubMed
LinkOut - more resources
Full Text Sources

