The Influence of Age on the Effective Dosage of Intravenous Remimazolam for the Relief of Preoperative Anxiety in Pediatric Patients at Median and 95% Effective Doses: A Prospective Study
- PMID: 40486122
- PMCID: PMC12140925
- DOI: 10.2147/DDDT.S515924
The Influence of Age on the Effective Dosage of Intravenous Remimazolam for the Relief of Preoperative Anxiety in Pediatric Patients at Median and 95% Effective Doses: A Prospective Study
Abstract
Purpose: Preoperative anxiety is an urgent problem in pediatric patients. This trial evaluated intravenous remimazolam for preoperative sedation in pediatric patients, assessing efficacy, safety, and age-dependent dose effects.
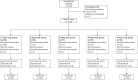
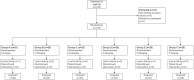
Patients and methods: In this two-part study, Aged 1~6 years old, 293 ASA I-II children [Parental Separation Anxiety Score (PSAS) ≥3 after nonpharmacological interventions] were enrolled. Part I: children were divided into 5 groups according to their age, and the trial was conducted by the Dixon-Massey sequential method. The first child in each group received a dose of 0.3 mg/kg of remimazolam, with a drug dose gradient of 0.05 mg/kg. Part II: 150 children were randomly selected and assigned to receive remimazolam 0.2-0.3 mg/kg. The main observations of this study were sedation effect and safety.
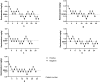
Results: The ED50 and 95% confidence interval (CI) for children aged 1-2 years was 0.14 (0.11-0.16) mg/kg, for children aged 2-3 years was 0.14 (0.11-0.17) mg/kg, for children aged 3-4 years was 0.16 (0.12-0.19) mg/kg, and for children aged 4-5 years was 0.14 (0.11-0.16) mg/kg, 5-6 years 0.13 (0.10-0.16) mg/kg, with no significant difference between age groups (P=0.525). The ED95 for preoperative sedation in children aged 1-6 years was 0.29 mg/kg (95% CI: 0.27-0.40). The difference in MOAA/S scores between the different dose groups in Part II was statistically significant (p<0.001) at 2 minutes after dosing. None of the adverse events that occurred after the use of remimazolam in this trial required the use of medication for intervention.
Conclusion: Remimazolam can be effectively used for preoperative sedation in children aged 1-6 years with low circulatory and respiratory effects, and there was no difference in the effective dose of the drug by age.
Keywords: dose-effect relationship; drug; pediatric patient; sedation with wakefulness.
© 2025 Chen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Remimazolam for successful sedation in children with left-to-right shunt congenital heart disease: An up-and-down sequential allocation trial.Eur J Anaesthesiol. 2025 Aug 1;42(8):697-703. doi: 10.1097/EJA.0000000000002183. Epub 2025 Apr 4. Eur J Anaesthesiol. 2025. PMID: 40223488 Free PMC article.
-
ED50 and ED95 of remimazolam for loss of consciousness in young children: a dose-finding study for induction of anaesthesia.Br J Anaesth. 2025 Jun;134(6):1709-1716. doi: 10.1016/j.bja.2025.02.004. Epub 2025 Mar 18. Br J Anaesth. 2025. PMID: 40107902 Clinical Trial.
-
Effect of Fentanyl on Remimazolam-Induced Sedation in Female Patients Undergoing Hysteroscopic Surgery: A Randomized Controlled Trial.Drug Des Devel Ther. 2025 Feb 27;19:1393-1401. doi: 10.2147/DDDT.S504189. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40034404 Free PMC article. Clinical Trial.
-
Heliox for croup in children.Cochrane Database Syst Rev. 2021 Aug 16;8(8):CD006822. doi: 10.1002/14651858.CD006822.pub6. Cochrane Database Syst Rev. 2021. PMID: 34397099 Free PMC article.
-
Pharmacological treatment of gastro-oesophageal reflux in children.Cochrane Database Syst Rev. 2023 Aug 22;8(8):CD008550. doi: 10.1002/14651858.CD008550.pub3. Cochrane Database Syst Rev. 2023. PMID: 37635269 Free PMC article.
References
-
- Coté CJ. Sedation for the pediatric patient. A review. Pediatr Clin North Am. 1994;41(1):31–58. - PubMed
-
- Zhang MQ, Xu MZ, He Y, Su YW, Ma J, Zuo YX. Comparison of S-ketamine and midazolam for intravenous preoperative sedative and anxiolytic effects in preschool children: study protocol for a randomized controlled clinical trial. Trials. 2023;24(1):724. doi:10.1186/s13063-023-07767-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical