hiPSC-derived cardiac fibroblasts dynamically enhance the mechanical function of hiPSC-derived cardiomyocytes on an engineered substrate
- PMID: 40486207
- PMCID: PMC12141862
- DOI: 10.3389/fbioe.2025.1546483
hiPSC-derived cardiac fibroblasts dynamically enhance the mechanical function of hiPSC-derived cardiomyocytes on an engineered substrate
Abstract
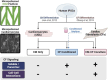
Introduction: Cardiac fibroblasts deposit and turnover the extracellular matrix in the heart, as well as secrete soluble factors that play critical roles in development, homeostasis, and disease. Coculture of CFs and human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (CMs) enhances CM mechanical output, yet the mechanism remains unclear.
Methods: Here, we use an in vitro engineered platform to compare the effects on CM mechanical function of direct CM-CF Coculture and soluble signaling alone through CF Conditioned Medium to a CM Only monoculture. Mechanical analysis is performed using digital image correlation and custom software to quantify the coordination and organization of CM contractile behavior.
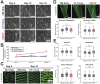
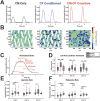
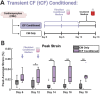
Results: CM-CF Coculture induces larger CM contractile strains, and an increased rate of spontaneous contraction compared to CM Only. Additionally, CM-CF Cocultures have increased contractile anisotropy and myofibril alignment and faster kinetics. The paracrine effects of fibroblast conditioned medium (FCM) are sufficient to induce larger contractile strains and faster contraction kinetics with these effects remaining after the removal of FCM. However, FCM does not influence CM spontaneous rate, contractile alignment, anisotropy, or relaxation kinetics compared to CM Only control.
Discussion: These data suggest that hiPSC-CFs exert dynamic and multifactorial effects on the mechanical function of hiPSC-CMs and highlight the importance of CFs in both the native heart and in vitro cardiac models. Further, this work demonstrates the applicability of the coculture-conditioned medium-monoculture paradigm to decouple the effects of paracrine factor and cell-cell signaling on hiPSC-CM mechanical function and maturation.
Keywords: cardiac fibroblasts; cardiomyocytes; hiPSC; mechanical function; tissue engineering.
Copyright © 2025 Josvai, Lawson, Kanade, Kalluri, Anderson, Zhang, Stempien, Eckhardt, Kamp and Crone.
Conflict of interest statement
TK is a consultant for Fujifilm Cellular Dynamics Incorporated. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Integration of co-culture conditions and 3D gelatin methacryloyl hydrogels to improve human-induced pluripotent stem cells-derived cardiomyocytes maturation.Front Bioeng Biotechnol. 2025 Jul 14;13:1576824. doi: 10.3389/fbioe.2025.1576824. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40727646 Free PMC article.
-
Generation of a human iPSC-derived cardiomyocyte/fibroblast engineered heart tissue model.F1000Res. 2024 Feb 12;12:1224. doi: 10.12688/f1000research.139482.2. eCollection 2023. F1000Res. 2024. PMID: 38298530 Free PMC article.
-
Stimulating Calcium Handling in hiPSC-Derived Engineered Cardiac Tissues Enhances Force Production.Stem Cells Transl Med. 2022 Mar 3;11(1):97-106. doi: 10.1093/stcltm/szab002. Stem Cells Transl Med. 2022. PMID: 35641165 Free PMC article.
-
Human iPSC-Cardiomyocytes as an Experimental Model to Study Epigenetic Modifiers of Electrophysiology.Cells. 2022 Jan 7;11(2):200. doi: 10.3390/cells11020200. Cells. 2022. PMID: 35053315 Free PMC article. Review.
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

