The Flipons, Infections, and Amyloids that Foreshadow the Fading Memories of Alzheimer's Disease
- PMID: 40486248
- PMCID: PMC12144360
- DOI: 10.1177/26331055251338815
The Flipons, Infections, and Amyloids that Foreshadow the Fading Memories of Alzheimer's Disease
Abstract
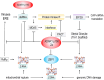
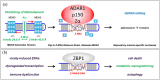
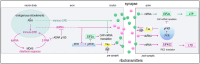
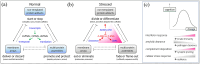
Our memories are almost magical. We can experience an event for a short moment in time and quickly recall it decades later. This review explores the impact of some relatively new discoveries in the field of flipon biology that provide insight into diseases associated with impaired memory function. I examine how an ancient immune system based on Z-DNA and Z-RNA (collectively called ZNAs) regulates pathways that impact the memories modeled by synapses. The outcomes depend on intracellular defenses activated by endogenous retroelements (ERE) and virus, and on extracellular responses to ZNAs in bacterial biofilms. The bacterial amyloids and complement activation pathways further exacerbate the decline of cognitive and affective functions by inducing remodeling of synapses. In addition to immune EREs, a class of memory EREs potentially acts as ribotransmitters. These RNAs are transported across the synapse to program the connections between neurons that underlie the formation and remodeling of memories. Examples exist of ribotransmitters derived from ERE transcripts and assembled into capsids capable of transsynaptic transmission. In contrast, the immune EREs protect the nervous system by dismantling synapses to prevent viruses and retrotransposons from crossing them. The complexity of the interactions between memory and immune EREs likely give rise to the inverted U-shaped dose-response curves for the therapeutics currently available to treat cognitive decline. Other approaches for disease prevention are suggested, along with those that promote the regeneration and reprogramming of neuronal circuits.
Keywords: Alzheimer’s disease; Flipons; Z-DNA and Z-RNA; bacterial infection; complement; endogenous retroelements; viruses.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Hebb DO. The Organization of Behavior; A Neuropsycho-logical Theory. A Wiley book in clinical psychology. Wiley; 1949:xix, 335 p.
-
- Pribram KH, Nuwer M, BaronN RJ. The holographic hypothesis of memory structure in brain function and perception. In: Krantz DH, ed. Contemporary Developments In Mathematical Psychology. W. H. Freeman; 1974:416-457.
-
- Falconer KJ, Grimmett GR. On the geometry of random Cantor sets and fractal percolation. J Theor Probab. 1992;5(3):465-485. doi: 10.1007/bf01060430 - DOI
-
- Ferreirós Domínguez J. Labyrinth of Thought: A History of Set Theory and Its Role in Modern Mathematics. 2nd rev. ed. Birkhäuser; 2007:xxv, 466 p.
Publication types
LinkOut - more resources
Full Text Sources

