Lenvatinib versus bevacizumab when combined with PD-1/L1 inhibitor and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma
- PMID: 40486515
- PMCID: PMC12141330
- DOI: 10.3389/fimmu.2025.1573098
Lenvatinib versus bevacizumab when combined with PD-1/L1 inhibitor and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma
Abstract
Introduction: The combination of anti-angiogenic agents, PD-1/L1 inhibitors, and hepatic arterial infusion chemotherapy (HAIC) has emerged as an important strategy for unresectable hepatocellular carcinoma (uHCC), yet comparative data on efficacy and safety between different anti-angiogenic agents (lenvatinib [LenHAP] or bevacizumab [BevHAP]) remain lacking, especially in patients with potential resectable features (PotenR).
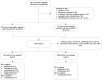
Methods: This retrospective study included patients from 3 hospitals. Included patients received LenHAP or BevHAP as the first-line treatment. The overall survival (OS), progression-free survival (PFS), objective response rate (ORR), conversion resection rate (CRR) and adverse events (AE) were compared.
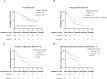
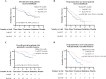
Results: We included 108 uHCC patients in each group after propensity score matching (PSM), of which PotenR patients accounted for 34.3%. Compared with BevHAP group, the LenHAP group demonstrated significantly prolonged median PFS (12.6 vs. 8.1 months; HR, 0.64; 95% CI, 0.46-0.90; p=0.0085), with a trend toward improved OS (26.4 vs. 19.6 months; HR, 0.71; 95% CI, 0.41-1.1; p=0.091). PotenR patients receiving LenHAP achieved superior outcomes, including markedly extended OS (both not reached in median, p=0.018), PFS (19.8 vs. 11.5, months, p=0.0067), and higher conversion resection rates (52.6% vs. 25.0%, p=0.015). Both regimens showed comparable safety profiles, with similar frequencies of grade 3-4 adverse events (47.2% vs. 39.8%, p=0.27) and serious adverse events (4.6% vs. 8.3%, p=0.27).
Conclusions: LenHAP might offer enhanced clinical benefits over BevHAP in uHCC, particularly for PotenR patients, while maintaining equivalent tolerability.
Keywords: bevacizumab; combination therapy; hepatocellular carcinoma; lenvatinib; potential resectable.
Copyright © 2025 Huang, Xu, Liu, Chen, Wu, Li, Lu, Wei, Zhang, Chen, Xu, Shi and Lai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Conversion study of hepatocellular carcinoma using HAIC combined with lenvatinib and PD-1/L1 immunotherapy under the guidance of BCLC staging.Front Immunol. 2025 Jun 2;16:1596864. doi: 10.3389/fimmu.2025.1596864. eCollection 2025. Front Immunol. 2025. PMID: 40529364 Free PMC article.
-
Lenvatinib combined with anti-PD-1 antibodies plus locoregional treatment for initial unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a multicenter real-world study.BMC Cancer. 2025 Jul 10;25(1):1162. doi: 10.1186/s12885-025-14543-9. BMC Cancer. 2025. PMID: 40640796 Free PMC article.
-
Hepatic arterial infusion chemotherapy combined with lenvatinib and immune checkpoint inhibitor versus lenvatinib for advanced hepatocellular carcinoma: a multicenter study with propensity score and coarsened exact matching.Radiol Med. 2025 May;130(5):662-673. doi: 10.1007/s11547-025-01975-3. Epub 2025 Mar 12. Radiol Med. 2025. PMID: 40072804 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Efficacy and safety of lenvatinib plus transarterial chemoembolization with or without programmed death-1 inhibitors in the treatment of intermediate or advanced hepatocellular carcinoma: a systematic review and meta-analysis.Front Immunol. 2025 Jul 24;16:1586914. doi: 10.3389/fimmu.2025.1586914. eCollection 2025. Front Immunol. 2025. PMID: 40777037 Free PMC article.
References
-
- Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. . Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

