First-in-human evaluation of a no-alpha interleukin-2 mutein: safety and preliminary pharmacodynamic and clinical effect
- PMID: 40486521
- PMCID: PMC12141302
- DOI: 10.3389/fimmu.2025.1589042
First-in-human evaluation of a no-alpha interleukin-2 mutein: safety and preliminary pharmacodynamic and clinical effect
Abstract
Introduction: Interleukin 2 (IL-2) is essential for immune system activation. To reduce toxicity and prevent the activation of regulatory T cells (T-regs), a novel IL-2 variant containing 4-point mutations that prevent its interaction with the alpha chain of the receptor was designed. In preclinical studies, the no-alpha mutein preferentially stimulate CD8-T cells and natural killer (NK) cells compared to Tregs. Mutein also showed greater antitumor capacity than the native molecule in several tumor models.
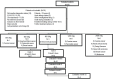
Methods: Patients with advanced solid tumors were included in a single-arm dose-escalation Phase I trial. The objectives of this study were to evaluate the safety and identify the recommended phase 2 dose. The effects on the most important immune subpopulations and preliminary objective response were also assessed. The protocol was listed in the National Registry for Clinical Trials (https://rpcec.sld.cu/ensayos/RPCEC00000234-En).
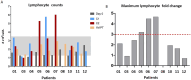
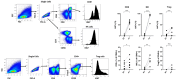
Results and discussion: In this phase I trial, 13 patients with advanced cancer were treated with five dose levels of IL-2 mutein, from 300 to 2400 IU/kg. The treatment was safe, and the maximum tolerated dose was not reached. Dose escalation did not continue, as a greater clinical and pharmacodynamic effect was observed at intermediate doses. One patient developed a possibly related serious event consisting on ventricular dysfunction and pneumonitis. No toxic deaths or vascular leak syndromes were detected, and the most frequent toxicities were chills, fever, and tachycardia. After treatment, most patients experienced an expansion of the total lymphocyte counts and the CD8-T cells and NK cells.
Clinical trial registration: https://rpcec.sld.cu/ensayos/RPCEC00000234-En, identifier RPCEC00000234.
Keywords: cancer immunotherapy; first-in-human; interleukin-2; mutein; phase I.
Copyright © 2025 Caballero Aguirrechu, Mestre Fernández, Soriano García, Lim Alonso, Soto García, Fleites Calvo, Mariño de la Puente, Vega Carvajal, Ávila Pérez, Mendoza Hernández, García López, Tarinas Reyes, García-Pérez, Díaz Borges, Ledón Naranjo, Lozada Chang, García Vega, Alvárez Lobaina, Alvárez Cardona, Lorenzo-Luaces Alvárez, Crombet Ramos, Carmenate Portilla and León Monzón.
Conflict of interest statement
Eleven authors GG, CD, NLN, SL, YG, AA, MA, PL-L, TCR, TCP, and KL worked at the Center of Molecular Immunology, the institution that patented and manufactured the IL-2 mutein. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care.Cochrane Database Syst Rev. 2018 Jun 5;6(6):CD006332. doi: 10.1002/14651858.CD006332.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Sep 15;9:CD006332. doi: 10.1002/14651858.CD006332.pub4. PMID: 29869799 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Claudin 18.2-targeting antibody-drug conjugate CMG901 in patients with advanced gastric or gastro-oesophageal junction cancer (KYM901): a multicentre, open-label, single-arm, phase 1 trial.Lancet Oncol. 2025 Feb;26(2):227-238. doi: 10.1016/S1470-2045(24)00636-3. Epub 2025 Jan 6. Lancet Oncol. 2025. PMID: 39788133 Clinical Trial.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

