Safety, efficacy, and quality of life with cemiplimab treatment among non-small cell lung cancer patients: a systematic review and meta-analysis
- PMID: 40486556
- PMCID: PMC12140711
- DOI: 10.1097/MS9.0000000000003329
Safety, efficacy, and quality of life with cemiplimab treatment among non-small cell lung cancer patients: a systematic review and meta-analysis
Abstract
Background: Immune checkpoint inhibitors have shown promise in treating advanced non-small cell lung cancer and have a solid safety profile. Cemiplimab can be used as monotherapy or in combination with chemotherapy for both squamous and non-squamous cancers. We opted for a systematic review and meta-analysis to find out the efficacy outcome and safety profile along with quality-of-life data of cemiplimab for advanced non-small-cell lung cancer (NSCLC).
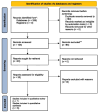
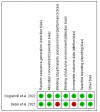
Methods: A rigorous search of literature on PubMed, Embase, and Google Scholar was done to find relevant published publications till December 1, 2023. Outcomes like objective response rate (ORR), overall survival (OS), progression-free survival (PFS), common side effects, and quality of life among the cemiplimab and the control group were used to conduct meta-analysis using the fixed/random effect model for combined odds ratio (OR), combined hazard ratio (HR) and mean difference at confidence interval (CI) of 95%.
Results: Two randomized clinical trials EMPOWER 1 and EMPOWER 3 with 1092 advanced NSCLC patients along with their follow-up studies were included. There was significantly higher OS (HR = 0.62 [0.54, 0.70], P < 0.0001) and PFS (HR = 0.54 [0.48, 0.60], P < 0.0001] in the treatment group. Similarly, the combined ORR was significantly higher in the treatment group as compared to the control group (2.63 [95% CI: 2.17-3.20, P ≤ 0.001]). Treatment-emergent adverse effects were not different between the groups. Finally, the quality-of-life scores between the two groups were nonsignificant.
Conclusion: With regard to OS, PFS, ORR, quality of life scores, and an acceptable safety profile in advanced non-small cell lung cancer, cemiplimab showed clinically relevant and statistically significant improvements making it standard of care for such patients.
Keywords: cemiplimab; chemotherapy; non-small cell lung cancer.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article. None to be disclosed by any author.
Figures
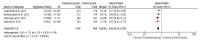

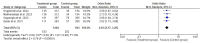

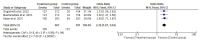
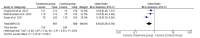

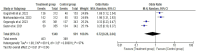
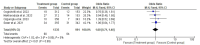

Similar articles
-
Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ⩾50.Ther Adv Med Oncol. 2022 Jun 16;14:17588359221105024. doi: 10.1177/17588359221105024. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35747163 Free PMC article.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis.Ther Adv Chronic Dis. 2023 Oct 11;14:20406223231189224. doi: 10.1177/20406223231189224. eCollection 2023. Ther Adv Chronic Dis. 2023. PMID: 37841212 Free PMC article.
-
First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2023 Sep;24(9):989-1001. doi: 10.1016/S1470-2045(23)00329-7. Epub 2023 Aug 14. Lancet Oncol. 2023. PMID: 37591293 Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
