CDK1-mediated phosphorylation of USP37 regulates SND1 stability and promotes oncogenesis in colorectal cancer
- PMID: 40486858
- PMCID: PMC12138069
- DOI: 10.1016/j.apsb.2025.02.014
CDK1-mediated phosphorylation of USP37 regulates SND1 stability and promotes oncogenesis in colorectal cancer
Abstract
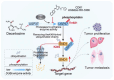
Colorectal cancer (CRC) poses a severe global health challenge with high incidence and mortality rates. USP37 has been identified as the bona fide deubiquitinase of SND1, playing a critical role in stabilizing SND1, thereby augmenting its oncogenic potential. The interaction between USP37 and SND1 was confirmed through extensive proteomics, ubiquitinomics, and interactomics, underscoring their synergistic effects on CRC proliferation and metastasis. Additionally, CDK1 has emerged as a pivotal regulator of USP37, phosphorylating it at threonine 631 rather than serine 628, enhancing its deubiquitinase activity, and consequently stabilizing SND1 to drive CRC malignancy further. Histological analyses of human CRC samples linked the upregulation of CDK1 and USP37 with increased SND1 levels and poor patient prognosis. High-throughput virtual screening and subsequent experimental validation identified Dacarbazine as a pharmacological inhibitor of USP37, and its inhibition disrupted SND1 stability, hindering CRC cell proliferation and metastasis. This study reveals a novel and promising molecular mechanism driving CRC progression through the CDK1-USP37-SND1 axis, highlighting the clinical importance of targeting this pathway to improve patient outcomes.
Keywords: CDK1; Colorectal cancer; Dacarbazine; Deubiquitination; Oncogenesis; Phosphorylation; SND1; USP37.
© 2025 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures








References
-
- Morgan E., Arnold M., Gini A., Lorenzoni V., Cabasag C.J., Laversanne M., et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–344. - PubMed
-
- Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–685. - PubMed
-
- Dewson G., Eichhorn P.J.A., Komander D. Deubiquitinases in cancer. Nat Rev Cancer. 2023;23:842–862. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

